According to the World Health Organisation the AD prevalence equals 5.5 % above 60 year of age and increases for elderly people (clinical AD: 16% 85y, 22% 90y). Thus age is the dominant risk factor overruling even the positive impacts of nutrition and education. Despite all efforts, the exact cause of Alzheimer's disease is still unknown, although a number of factors have been suggested. These include the metabolism and regulation of the amyloid precursor protein, plaque-related proteins, tau proteins, zinc, copper and aluminium cations.
Acetyl cholinesterase inhibitors and general therapy moderate symptoms at the onset of the disease, they improve cognitive function, as expressed in Alzheimer’s Disease Assessment Scale (ADAS-COG), but these drugs do not address the severe mortality at the final stage. Promising results were obtained with nonsteroidal anti-inflammatory drugs (NSAID). Immunization therapies against Ab hold high potential and are under investigation by several companies.
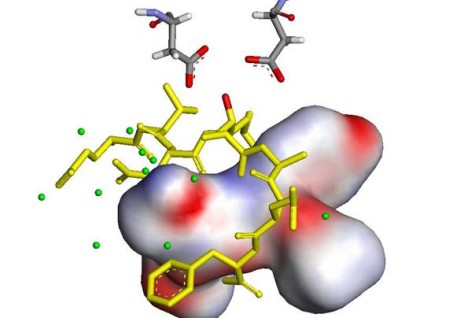
Gene mutations linked to early-onset Alzheimer’s disease afflicted families in London and Sweden and additional polymorphisms, that either cause or further AD, provided some insight into the biological pathways and the involvement of the amyloid precursor protein (APP). A rational approach to a successful, causal therapy is based on a detailed understanding of Ab formation, deposition and the inflammatory consequences. Decisive functions were assigned to the amyloid precursor protein (APP) and its degrading aspartic proteases: β-secretase (BACE) and the presenilins (PS is also called ϒ-secretase), which are in the focus of our research efforts.
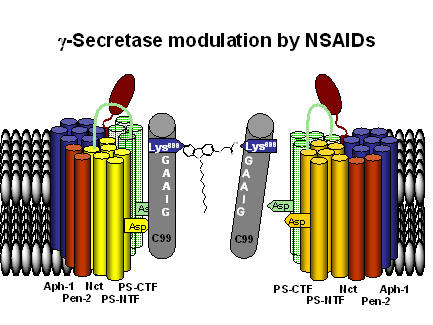
The current hypothesis for the modulation of ϒ-secretase is depicted below. The modulator binding to the substrate C99 interferes with substrate dimerization. The monomeric substrate is then metabolized to non-pathological fragments.
This is a simplified cartoon of the APP processing. The up to 771 amino acid long APP, includes a signalling sequence, a large extramembraneous sequence and the crucial membrane spanning domain followed by a short cytoplasmic tail. The nonpathological cleavage occurs between Lys687-Leu688 by the a-secretase. This dominating event leaves just 10% of the APP behind for the β-secretase, which is one of the two culprits in amyloid-β-formation. The group at the TU Darmstadt develops inhibitors of β-secretase and selective modulators of ϒ-secretase.
The current hypothesis for the modulation of ϒ-secretase is depicted below. The modulator binding to the substrate C99 interferes with substrate dimerization. The monomeric substrate is then metabolized to non-pathological fragments.
Documents for Alzheimer Research
Amyloid ß and tau in Alzheimer's disease (Poster pdf F. La Ferla, Wyeth/Elan) (opens in new tab)
Molecular Basis of Alzheimer's Disease (P.C. Fraering on Youtube)
3sat Interview mit M. Madeja: Alt und vergesslich – wird Alzheimer zur Volkskrankheit?
Intramembrane Proteolysis: Michael S. Wolfe, Chem. Reviews 2009