58
R. Ghazali, E. Schick, D. Didier*
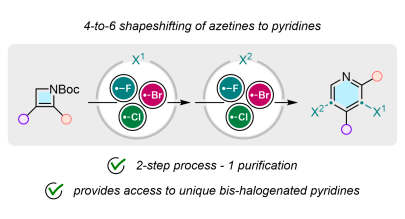
4-to-6 Skeletal Editing of Azetines to Bis-Halogenated Pyridines
ChemRxiv 2025
57
E. Friedrich, P. Freund, C. Lorenz, T. Heinrich, D. Didier, M. Reggelin*
Synthesis of Sulfondiimidoates by Desoxychlorination of Sulfonimidamides
Org. Lett. 2025, accepted
56
M. Cedzich, B. Boutet, F. Rambaud,‡ E. Friedrich,‡ S. Scholz, D. Didier*
(‡ equal contributions)
Synthesis of Bis-arylated Squaryls via Addition of Organocopper Species onto Squaryl Dichlorides
Eur. J. Org. Chem. 2025, asap
55
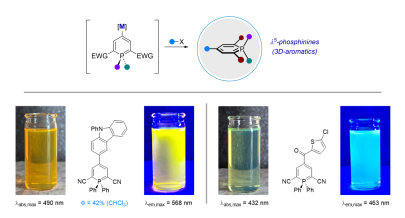
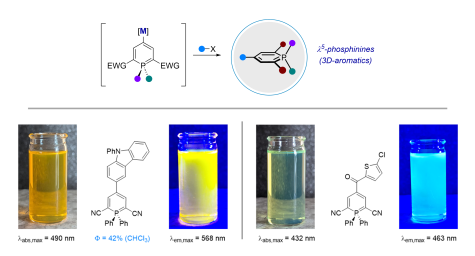
F. Rambaud, B. Takam-Fotie, R. Naumann, K. Heinze, D. Didier*
Functionalization of λ5-Phosphinines via Metalation Strategies – A New Route towards Fluorescent 3D-Aromatics
submitted
Archive version:
ChemRxiv 2025, DOI: 10.26434/chemrxiv-2025-r1h8f
54
R. Ghazali, E. Schick, S. Lèpre, J. Kranich, D. Didier*
Regiospecific Skeletal Editing of Azetines towards Halogenated Pyrroles
submitted
Archive version:
ChemRxiv. 2025, DOI: 10.26434/chemrxiv-2025-25grb
53
F. Trauner,‡ B. Boutet,‡ F. Rambaud,‡ V. N. Ngo, D. Didier*
(‡ equal contributions)
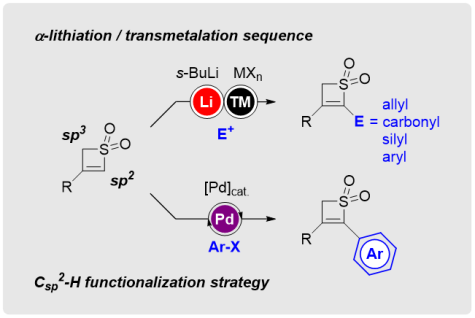
Diethylzinc Amylates: Selective Iodine–Zinc Exchange Reagents at Room Temperature
Org. Lett. 2025, asap
Archive version:
52
H. Chen, D. Wu, J. Holzinger, R. Götz, D. Didier, Anne K. Schütz, S. Schneider, P. Kielkowski*
Aryl Radicals Generated from Aryl Pinacol Boronates Modify Peptides and Proteins
Eur. J. Org. Chem. 2025, 28, e202401246
51
D. Didier,* B. Boutet
(book chapter)
Reference Module in Chemistry, Molecular Sciences and Chemical Engineering
Update 2025
Hydrozirconation of Alkenes and Alkynes
50
D. Didier,* F. Trauner, D. Jiang
(book chapter)
Science of Synthesis
Knowledge Updates 2025/1
9.8.17 Four-Membered Rings with One or More Heteroatoms (Update 2025)
- 2024 -
49
F. Trauner, B. Boutet, F. Pilz, V. Weber, D. Didier*
Zweifel olefination for C-glycosylation
Commun. Chem. 2024, 7, 306
DOI: 10.1038/s42004-024-01339-4
Archive version:
48
F. Trauner, R. Ghazali, J. Rettig, C. M. Thiele, D. Didier*
Stereoselectivepolar radical crossover for the functionalization of strained-ring systems
Commun. Chem. 2024, 7, 139
DOI: 10.1038/s42004-024-01221-3
Archive version:
- 2022 -
47
D. Didier*
short review (invited) Special Issue dedicated to Prof. Alain Krief
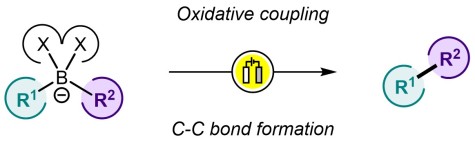
Forging C–C Bonds through Intramolecular Oxidative Coupling of Organoborates – An Overview
Synthesis 2023, 55, 232-239
DOI: 10.1055/a-1757-2680
46
F. Trauner,‡ F. Reiners,‡ K.-E. Apaloo-Messan, B. Nißl, M. Shahbaz, D. Jiang, J. Aicher, D. Didier*
(‡ equal contributions)
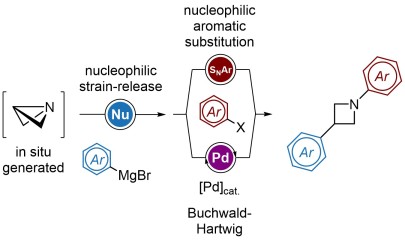
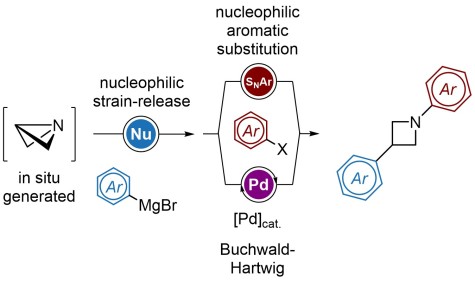
Strain-release arylations for the bis-functionalization of azetidines
Chem. Commun. 2022, 58, 2564-2567
DOI: 10.1039/d1cc07053c
Archive version:
45
F. Matz, A. Music, D. Didier, T.-C. Jagau*
Computational insights into electrochemical cross-coupling of quaternary borate salts
Electrochem. Sci. Adv. 2022, 2, e2100032
Archive version:
DOI:
10.26434/chemrxiv.14038736.v2 D O I: 10.26434/chemrxiv.14038736.v2
- 2021 -
44
F. Trauner, M. Shabhaz, D. Didier*
Wie der Würfel wirkt
Nach. Chem. 2021, 69 (Nov.), 72-75
43
D. Didier,* F. Reiners, A. N. Baumann
(book chapter)
Comprehensive Heterocyclic Chemistry IV
Volume 2, 2022,287-306
2.06 – Thietanes and Thietes: Monocyclic
42
D. Didier,* F. Reiners
Strategien bei der radikalischen Trifluoromethylierungen
Nach. Chem. 2021, 69 (Juli), 70-74
41
A. Music,† C. M. Nuber,† Y. Lemke, P. Spieß, D. Didier*
(† equal contributions)
Electro-alkynylation: Intramolecular Rearrangement of Trialkynylorganoborates for Chemoselective C(sp2)−C(sp) Bond Formation
Org. Lett. 2021, 23, 4179-4184
40
D. Didier,* F. Reiners
Uncommon Four-Membered Building Blocks – Cyclobutenes, Azetines and Thietes
Chem. Rec. 2021, 21, 1144-1160
39
D. Didier,* F. Reiners
Nazarov-Elektrocyclisierung: neue Twists für eine Lehrbuchreaktion
Nach. Chem. 2021, 69 (März), 72-75
38
A. Music, A. N. Baumann, F. Boser, N. Müller, F. Matz, T. C. Jagau, D. Didier*
Photocatalyzed Transition-Metal-Free Oxidative Cross-Coupling Reactions of Tetraorganoborates
Chem. Eur. J. 2021, 27, 4322-4326
Archive version:
ChemRxiv 2021, DOI: 10.26434/chemrxiv.13005509.v1
- 2020 -
37
F. Reiners, E. Joseph, B. Nißl, D. Didier*
Stereoselective Access to Azetidine-Based α‑Amino Acids and Applications to Small Peptide Synthesis
Org. Lett. 2020, 22, 8533-8537
DOI: 10.1021/acs.orglett.0c03131
Archive version:
ChemRxiv 2020, DOI: 10.26434/chemrxiv.12966803.v1
36
A. N. Baumann+, A. Music+, J. Dechent, N. Müller, T. C. Jagau, D. Didier*
(+ equal contributions)
Electro-Olefination—A Catalyst Free Stereoconvergent Strategy for the Functionalization of Alkenes
Chem. Eur. J. 2020, 26, 8382-8387
35
A. N. Baumann+, F. Reiners+, A. F. Siegle, P. Mayer, O. Trapp, D. Didier*
(+ equal contributions)
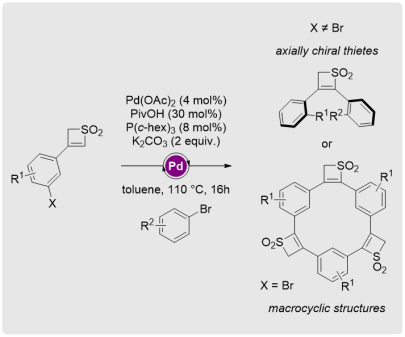
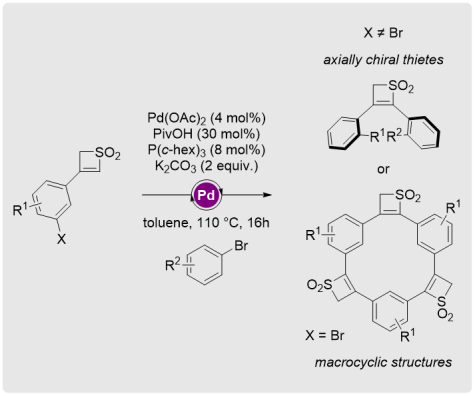
Thiete Dioxides as Templates Towards Twisted Scaffolds and Macrocyclic Structures
Chem. Eur. J. 2020, 26, 6029-6035
34
A. Music, A. N. Baumann, P. Spieß, A. Plantefol, T. C. Jagau, D. Didier*
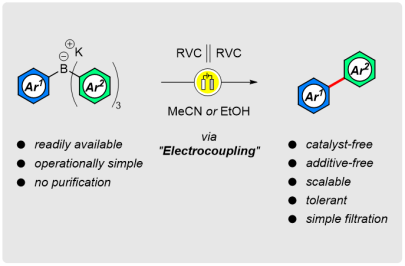
Electrochemical Synthesis of Biaryls via Oxidative Intramolecular Coupling of Tetra(hetero)arylborates
J. Am. Chem. Soc. 2020, 142, 4341-4348
DOI: 10.1021/jacs.9b12300
- 2019 -
33
A. Music, D. Didier*
Invited synopsis (Synpacts)
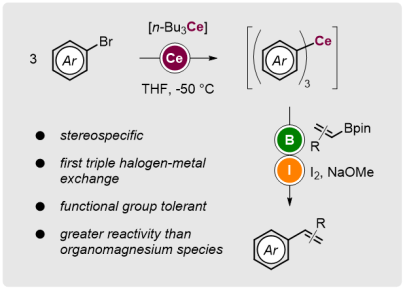
Organocerium: A New Contender for Halogen–Metal Exchanges
Synlett 2019; 30(16): 1843-1849
32
A. Music,† A. N. Baumann,† P. Spieß, N. Hilgert, M. Köllen, D. Didier*
(† equal contributions)
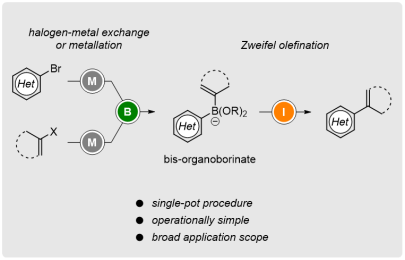
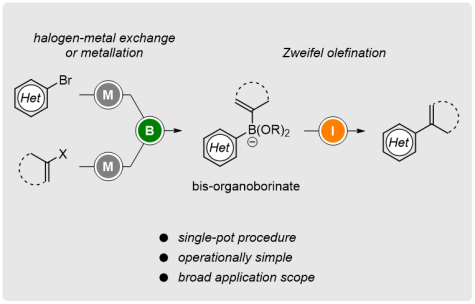
Single-Pot Access to Bisorganoborinates: Applications in Zweifel Olefination
Org. Lett. 2019, 21, 2189-2193
31
A. Music, C. Hoarau, N. Hilgert, F. Zischka, D. Didier*
Catalyst-Free Enantiospecific Olefination with In Situ Generated Organocerium Species
Angew. Chem. Int. Ed. 2019, 58, 1188-1192
- 2018 -
30
A. N. Baumann, F. Reiners, T. Juli, D. Didier*
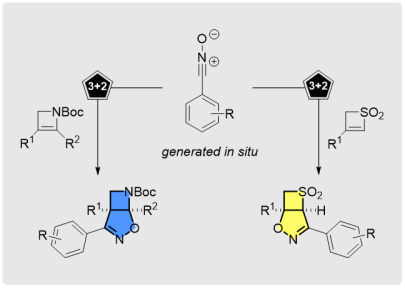
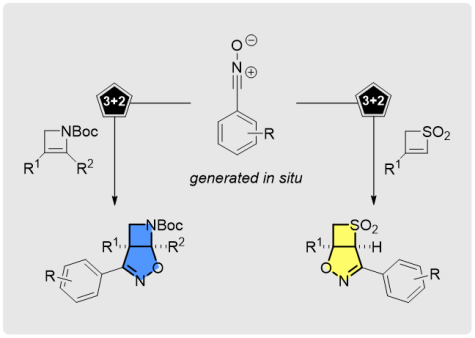
Chemodivergent and Stereoselective Access to Fused Isoxazoline Azetidines and Thietanes through [3+2]-Cycloadditions
Org. Lett. 2018, 20, 6736-6740
29
D. Didier,* A. N. Baumann, M. Eisold
invited “Digest paper” (review)
Unsaturated Four-Membered N-Heterocycles: From Synthesis to Applications
Tetrahedron Lett. 2018, 59, 3975-3987

28
M. Eisold,† A. Müller-Deku,† F. Reiners, D. Didier*
(† equal contributions)
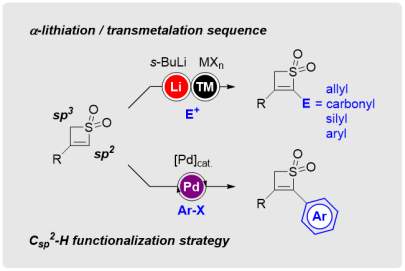
Parallel Approaches for the Functionalization of Thietes: α‑Metalation versus C−H Activation
Org. Lett. 2018, 20, 4654-4658
27
J. Boekhoven,* D. Didier*
Merging Art and Science – The 53rd Bgrgenstock Conference
conference report – invited
Angew. Chem. Int. Ed. 2018, 57, 10011-10014
26
A. N. Baumann,‡ Michael Eisold,‡ A. Music,‡ D. Didier*
(‡ equal contributions)
invited article as part of the Special Topic: Modern Coupling Approaches and their Strategic Applications in Synthesis
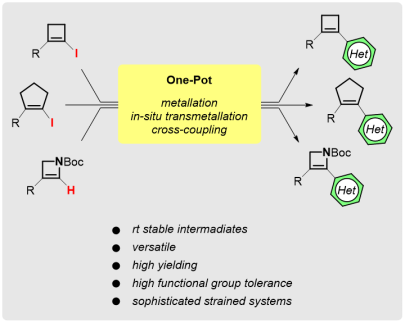
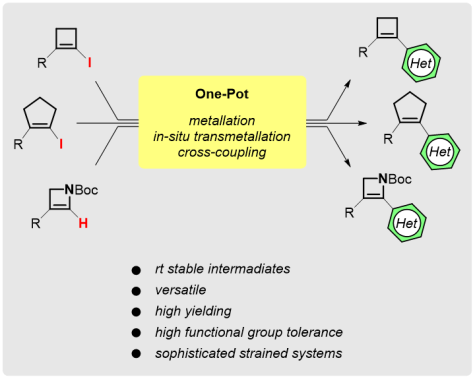
One-Pot Preparation of Stable Organoboronate Reagents for the Functionalization of Unsaturated Four- and Five-Membered Carbo- and Heterocycles
Synthesis 2018, 50, 3149-3160
25
A. N. Baumann, F. Schüppel, M. Eisold, A. Kreppel, R. de Vivie-Riedle,* D. Didier*
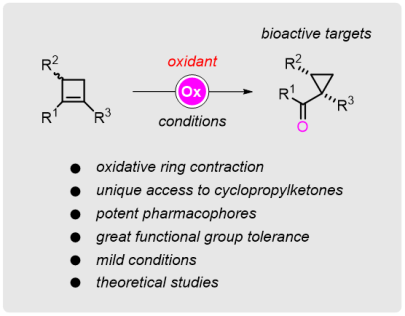
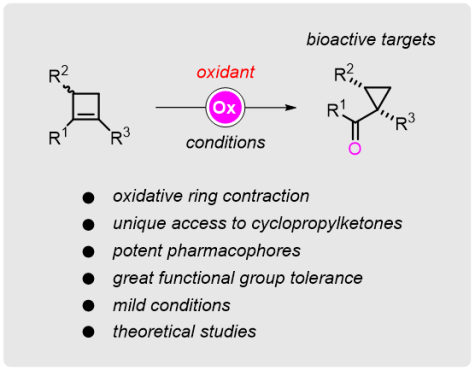
Oxidative Ring Contraction of Cyclobutenes: General Approach to Cyclopropylketones including Mechanistic Insights
J. Org. Chem. 2018, 83, 4905-4921
24
A. Music,† A. N. Baumann,† M. Eisold, D. Didier*
(† equal contributions)
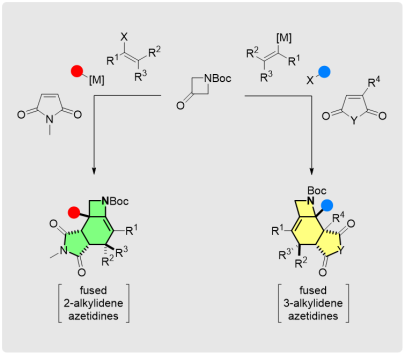
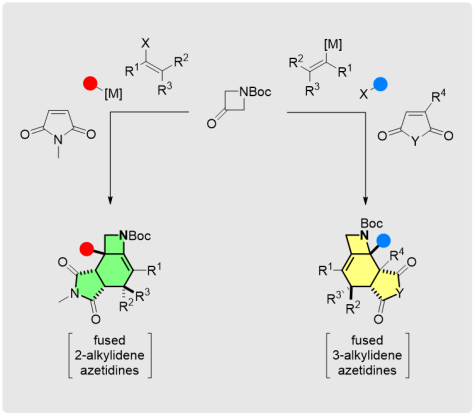
Regiodivergent Stereoselective Access to Fused Alkylideneazetidines
J. Org. Chem. 2018, 83, 783-792
- 2017 -
23
A. N. Baumann,∥ M. Eisold,∥ A. Music, G. Haas, Y. M. Kiw, D. Didier*
(∥ equal contributions)
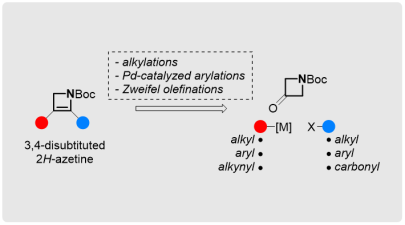
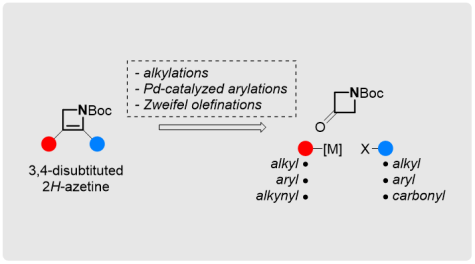
Methods for the Synthesis of Substituted Azetines
Org. Lett. 2017, 19, 5681-5684
22
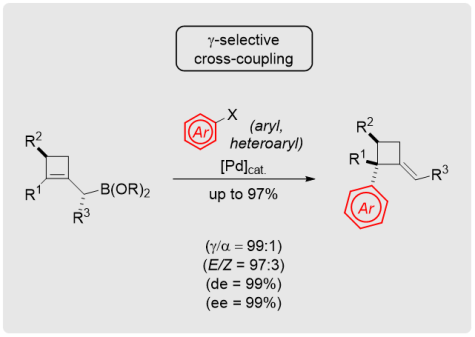
M. Eisold, D. Didier*
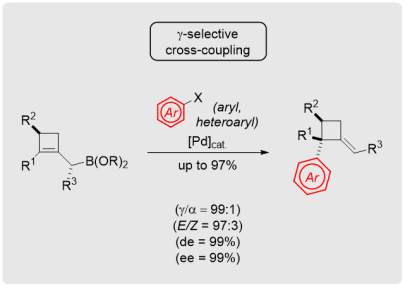
Stereoselective Access to Alkylidenecyclobutanes through γ‑Selective Cross-Coupling Strategies
Org. Lett. 2017, 19, 4046−-4049
21
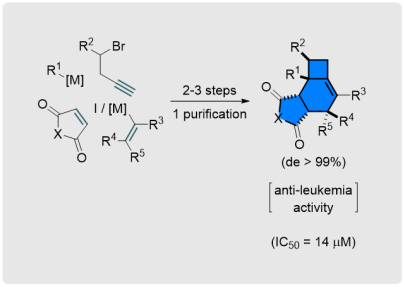
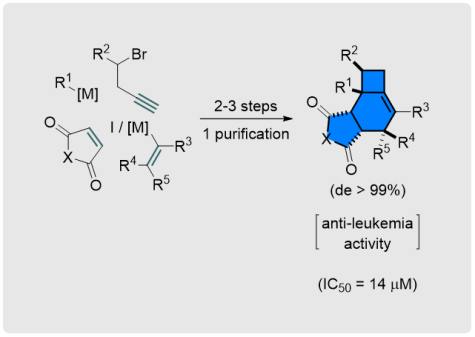
A. N. Baumann, M. Eisold, D. Didier*
Stereoselective Sequence toward Biologically Active Fused Alkylidenecyclobutanes
Org. Lett. 2017, 19, 2114-2117
20
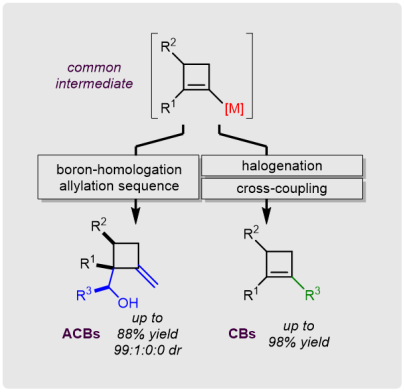
M. Eisold+, A. N. Baumann+, G. M. Kiefl, S. T. Emmerling, D. Didier*
(+ equal contributions)
Unsaturated Four-Membered Rings: Efficient Strategies for the Construction of Cyclobutenes and Alkylidenecyclobutanes
Chem. Eur. J. 2017, 23, 1634-1644
DOI : 10.1002/chem.201604585
- 2016 -
19
M. Eisold,‡ G. M. Kiefl,‡ D. Didier*
(‡ equal contributions)
Single-Pot Asymmetric Approach toward Enantioenriched Quaternary Stereocenter-Containing Alkylidenecyclobutanes
Org. Lett. 2016, 18, 3022-3025
18
A. N. Baumann, A. Music, K. Karaghiosoff and D. Didier*
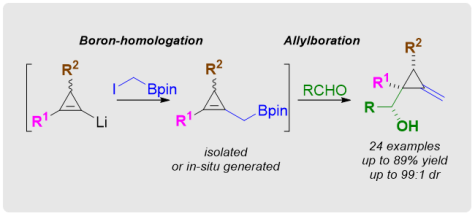
Highly Diastereoselective Approach to Methylenecyclopropanes via Boronhomologation/Allylboration Sequences
Chem. Commun. 2016, 52, 2529-2532
DOI: 10.1039/c5cc09904h
- 2015 -
17
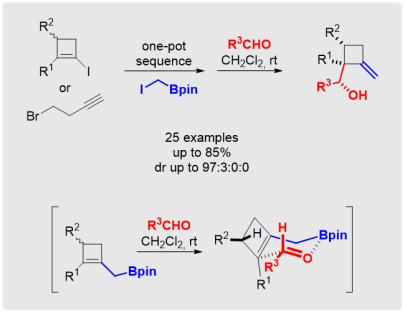
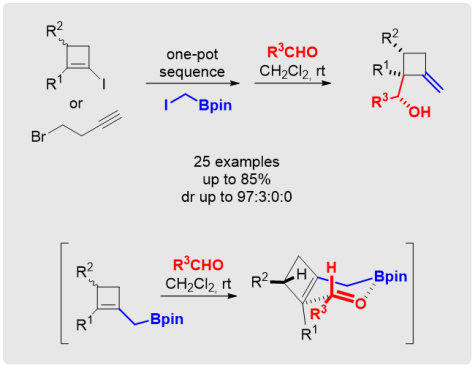
M. Eisold, D. Didier*
Highly Diastereoselective Synthesis of Methylenecyclobutanes by Merging Boron-Homologation and Boron-Allylation Strategies
Angew. Chem. Int. Ed. 2015, 54, 15884-15887