Biogenic Organogels: Sustainable Lubricating Grease Enhanced by Cellulose Derivatives
M.Sc. Linus Sprandl (since 2022)
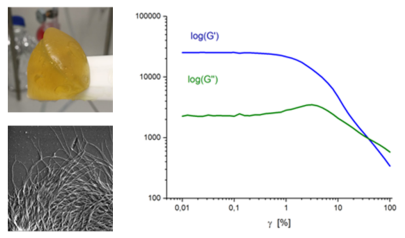
In a world where a substantial portion of our energy consumption is squandered through friction and wear processes, the quest for efficient lubricants becomes paramount. Not only can these lubricants significantly reduce production and transportation costs, but they also play a pivotal role in conserving precious resources and curbing harmful emissions. The traditional lubricants, primarily derived from mineral oil, and greases predominantly formulated with lithium soaps, have long been the industry norm. However, the escalating prices of these resources, particularly lithium, driven by the surging demand from Li-ion batteries, have made it imperative to seek alternative lubrication solutions, given the significant environmental impact associated with lithium production. Mining and processing lithium can have detrimental effects on local ecosystems and water resources.
Our project revolves around the development of biogenic organogels, which are materials formed by a network of organic molecules. We employ the power of biopolymers, specifically cellulose derivatives as gelling agents to create sustainable and biodegradable lubricating agents. Rheology techniques help us understand the behaviour of these biogenic organogels, allowing us to fine-tune formulations for optimized performance. Additionally, imaging procedures provide microscopic insights into how these agents interact within the lubricating grease.
Biomimetic plasma polymers for paper functionalization
M.Sc. Amelia Lösch-Zhang (2020 – 2024)
The project aims at creating new sustainable functional paper coatings to increase material performance and durability by imitating botanical protection strategies. This will be achieved by applying biogenic precursor molecules obtained from pulp production waste products to the paper surface with the assistance of plasma technology. Critical steps are the identification of suitable extractives and tuning of process parameters, as well as the analysis of resulting properties.
Lignin-barrier coatings for paper
M.Sc. Kerstin Bartels (2019 – 2023)
M.Sc. Enis Saritas (since 2022)
Due to our growing society and its environmental footprint, there is an increasing need for circular materials. In the future, there will be an increased demand for renewable resources. One common renewable resource is wood which leads to circular materials such as paper and lignin, a byproduct of paper production. Lignin has some very interesting and unique properties, that open up a wide field of research.
Biogenic wet strength agents based on cellulose derivates
M.Sc. David Seelinger (2019 – 2023)
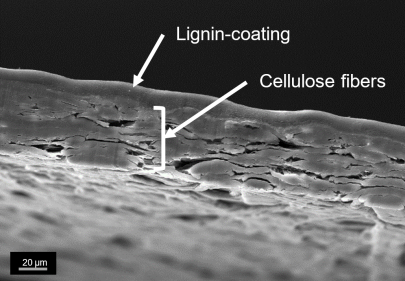
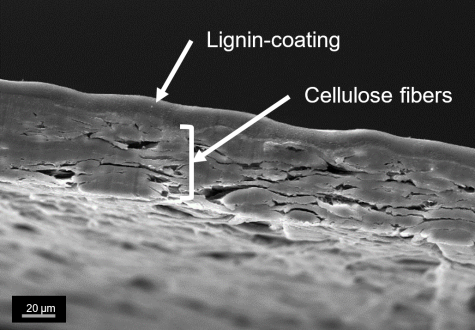
Established wet strength agents are based on petrochemicals and could raise environmental and health concerns. For this reason, there is a need to develop alternative wet strength agents based on renewable materials. An alternative approach is the functionalization and crosslinking of hydroxypropyl cellulose (HPC) via TEMPO-mediated oxidation. The crosslinking reaction occurs spontaneous at room temperature and mild pH-values, which allows the use of established paper additives. Oxidized HPC (keto-HPC) forms in the presence of industrial well-known paper additives such as polyethylene imine or biobased polysaccharides such as chitosan highly crosslinked systems, which greatly enhance the wet strength properties of paper. The utilization of such biobased wet strength agents could lead to new approaches in terms of recyclability and biodegradability.
Also, the further development of imaging methods for cellulose derivates via fluorescence microscopy provides a better understanding of the wet strength mechanism of those wet strength agents in paper. Keto-HPC also enables the formation of novel responsive hydrogels on a biogenic basis. In the future, such hydrogels could find application in sensors systems or agriculture due to their temperature-responsive properties.


Novel functional lignins as building blocks in preparation of polyurethane foams
M.Sc. Mareike Zieglowski (until 2020 )
Lignin, a bio-based polymer carrying an abundant number of alcoholic functions, can be used as a substitute for petrochemical products. At the moment, only a small amount of lignin is used for materials applications (about 2 %). Most of the Lignin is being burned for energy production. Lignin contains a large number of hydroxyl groups and it can be used as a macromonomer in polyurethane foam-formulations, as a low-cost polyol.
Polyurethane foams (PUs) are an important class of polymers, which are synthesized by a polyaddition reaction between polyols and polyisocyanate to form a urethanic linkage. The synthesis of such lignin-based PUs often follows two major strategies: (1) the Lignin is used directly without any previous chemical modification, with/without addition of further other polyols or (2) the lignin is chemically modified prior to the reaction to make hydroxyl functions available, which could increase the efficiency of binding the lignin into the PU polymer.
Derivatization of Lignin and its application in 2-component thermosets
Dr. Marcus Ott (until 2016)
In order to replace petroleum-based chemicals in the production of resins Lignin has been chemically modified. The strategy is the incorporation of chemically reactive groups onto the ligin.
For this, Kraft Lignin was aminated via Mannich reaction with various amines. In a subsequent step, a curing reaction was performed using commercially available Bisphenol-A epoxide and amine-terminated polypropylene glycol as hardener. With this strategy up to 30 w-% of lignin were successfully incorporated into the resin formulations. The samples were investigated by a set of analytical techniques such as ATR-IR, Elemental analysis, REM and mechanical analysis (3-point bending test according to DIN EN 178).
Comparison of the mechanical properties to neat resin showed, that resins containing 23 wt.-% modified lignin provided up to 74% of the flexural strength. Reference experiments with unmodified Lignin proved our hypothesis that the chemical modification of the lignin with amine groups has a significant impact on the reactivity during the curing reaction and therefore on the mechanical performance of the resulting resins.
Lignin-substituted bio-based epoxy resins
Dr.-Ing. Sabrina Mehlhase
Lignin as one of the most abundant renewable resources is an excellent raw material for several applications due to its highly functional character and source of aromatic building blocks. Taking advantage of its hydroxyl moieties, it can be used in epoxy-based thermosets as a feasible polymeric building block bringing new interesting features.
Epoxy-based thermosets are a very interesting class of polymers due to the adjustability of their properties which depend on the large variety of easy available starting materials and an adjustable cross-linking density. Incorporating lignin, either unmodified using lignin as filler, as accelerator through its hydroxyl moieties or functionalized as novel bio-based curing agents, highly lignin-substituted epoxy resins with promising properties can be achieved. Further, the substitution of Bisphenol-A based epoxy resins with bio-based alternatives such as epoxidized soybean or linseed oil is from great interest.
Value added chemical building blocks and lignin from wood
The use of renewable resources in the production of chemicals is in the perspective of sustainability highly desirable. It is also believed to create numerous societal benefits such as the revitalisation of rural areas. These drivers are increasingly recognized, especially by brand owners, thus creating a growing market pull for green products. At the same time biorefining and biochemicals processes become increasingly cost efficient. Consequently, chemicals produced from biomass feedstocks, including woody biomass, are considered to become a major growth area. In that context UPM, SEKAB, METabolic EXplorer and the Technische Universität Darmstadt have partnered, joining competences of forest, chemical and bio-technology industries.
The objective of ValChem is to demonstrate the technical and economic viability of an innovative and sustainable value chain, which utilises wood as raw material to produce the bio-alternative of the platform chemical monopropylenglykol as well as lignin-based performance chemicals.
Performance-lignin is intended to replace similar-in-application fossil components in high value added reactive resins and composites. This application will be developed jointly by the Technische Universität Darmstadt and UPM.
ValChem is a Demonstration Project and relates to the Bio Based Industries Joint Undertaking (BBI JU) annual work plan topic BBI Topic: BBI.VC1.D2 2014: “Chemical building blocks and value-added materials through integrated processing of wood”
The formal EU project info can be found in the CORDIS portal. For more information, please visit the ValChem-Website
Novel stimuli-responsive cellulose derivatives
M.Sc. Yonggui Wang (2013 – 2015)
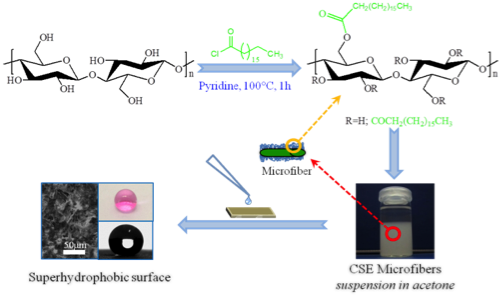
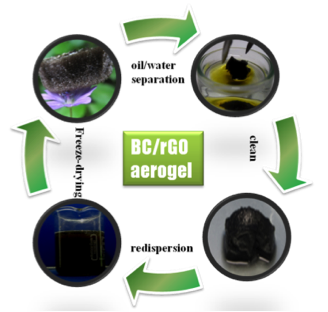
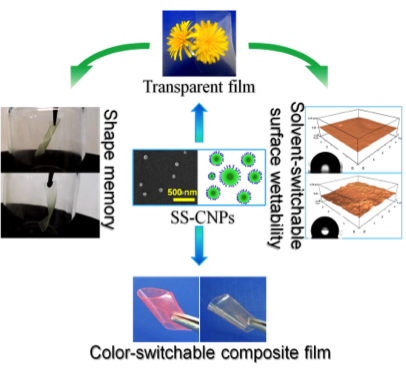
Cellulose derivatives have got a huge potential as sustainable, functional materials, which can substitute commercial, petro-chemical derived plastics. In this project, we are interested in the formation of novel cellulose-based stimuli-responsive materials, by functionalization of cellulose polymers with diverse groups. Examples progress from ultra-light nanocomposite aerogels of bacterial cellulose and reduced graphene oxide for specific absorption and separation of organic liquids, to superhydrophobic surfaces from surface-stearoylated cellulose fibers, and transparent, stimuli-responsive films from surface-stearoylated cellulose organogel nanoparticles.
Related Publications
[1] Wang, Y. G.; Yadav, S.; Heinlein, T.; Konjik, V.; Breitzke, H.; Buntkowsky, G.; Schneider, J. J.; Zhang, K., Ultra-light nanocomposite aerogels of bacterial cellulose and reduced graphene oxide for specific absorption and separation of organic liquids, Rsc Adv, 2014, 4, 21553-21558.
Self-assembling cyclic peptides as structure guides for nanostructured soft materials
Cyclic peptides exisiting of an alternating sequence of D- and L-amino acids possess a planar conformation, which allows theses molecules to form nanotubes by stacking onto each other. These rod-like nanoarchitectures are stabilized by inter-molecular hydrogen bonds. Interestingly, the residues of the amino acids point outwards and, hence, tailoring the chemistry of the outer surface of such peptide nanotubes is possible. In our research we attach initiator moieties to some of the amino acids and the latter are being used as initiation sites in controlled radical polymerization reactions, after self-assembling the initiator-modified peptide-rings.
The resulting “peptide-polymer hybrid nanotubes” (PPNT) exhibt a rod-like core-shell architecture, where a rigid core (peptide nanotube) is surrounded by a soft polymeric shell. Similar structures can be formed by first generating cyclo-peptide-polymer conjugates in solution, followed by a solvent-triggered self-assembly process in situ. The length and the diameter of PPNT seem to be governed by peptide-peptide as well as polymer excluded volume interactions, and can thus be controlled within certain limitations by adjusting the length of the peptide-attached polymer molecules.
Related Publications
[1] Couet, J.; Samuel, J. D. J. S.; Kopyshev; Santer, S.; Biesalski, M.: Angewandte Chemie-International Edition 2005 (44) 3297-3301.
[2] Couet, J.; M. Biesalski: Macromolecules 2006 (39) 7258-7268.
[3] Couet, J.; M. Biesalski: Small 2008 (4) 1008-1016.
[4] Loschonsky, S.; Couet, J.; Biesalski, M.: Macromol. Rapid Comm. 2008 (29) 309-315.
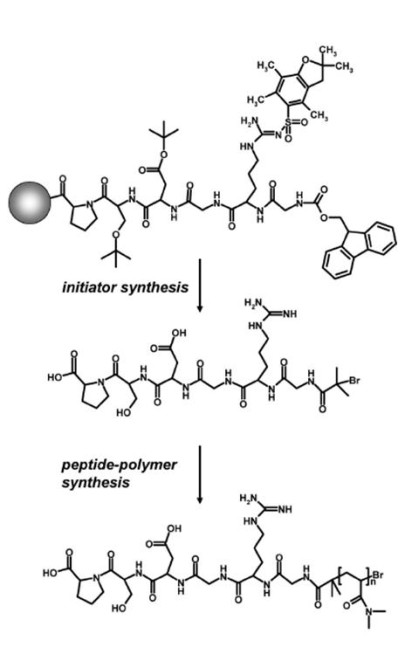
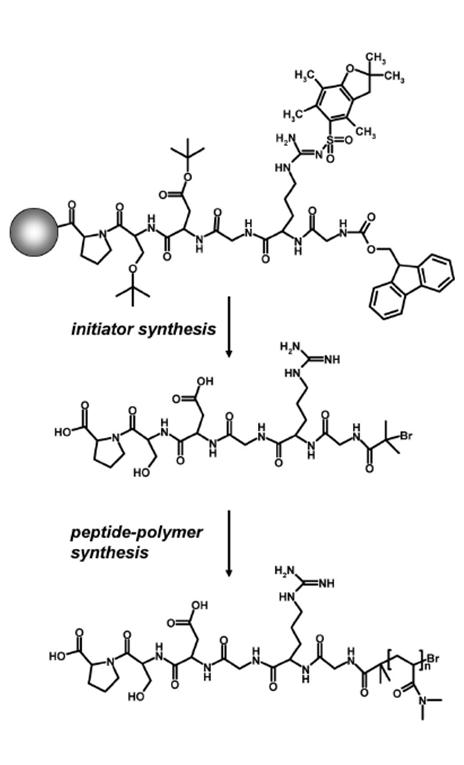
Biosynthetic peptides are being conjugated to synthetic polymers using both, divergent (i.e. where the polymer is prepared in situ using functional peptides as polymerization initiators or using peptides which are linked to polymerizable groups as monomers) as well as convergent strategies (i.e. linking pre-formed polymers to peptides). Peptide-polymers prepared by in situ polymerization include cyclo-peptide-polymers as outlined above, and bioactive peptide-polymers that interact with living cells in a desired fashion, as well as peptide-polymer materials that mimik mussel adhesive proteins. An example of a divergent synthesis of a biofunctional peptide-polymer conjugate is shown in the schematic illustration below. A cell-responsive peptide, carrying an ATRP initiator at the N-terminus is prepared by means of solid phase chemistry. This initator-modified peptide is then used to prepare peptide-polymers in situ. Molecular characteristics, such as the degree of polymerization, is controlled by adjusting appropriate polymerization conditions accordingly.
Despite modifying peptides with functional sites that can initiate a polymerization reaction, peptides can also be linked to functional groups that serve as a monomer species in a polymerization reaction. With respect to the latter, we have prepared so-called polymerizable peptide-amphiphiles, where functional peptides are linked to the polar head-group of a photo-polymerizable fatty acid. Examples include peptide-amphiphiles that carry a cell-responsive RGD-peptide as well as peptide-amphiphiles that are being modified with a functional ligand that is taken from the mussel-adhesive foot protein mefp-1 to generate adhesive nanocolloids. Again the synthesis is being performed by means of solid phase chemistry protocols.
Related Publications
[1] Biesalski, M.; Tu, R.; Tirrell, M. V.: Langmuir 2005 (21) 5663-5666.
[2] Loschonsky, S.; Shroff, K.; Worz, A.; Prucker, O.; Rühe, J.; Biesalski, M.:
Biomacromolecules 2008 (9) 543-552.