Welcome to the Reggelin group
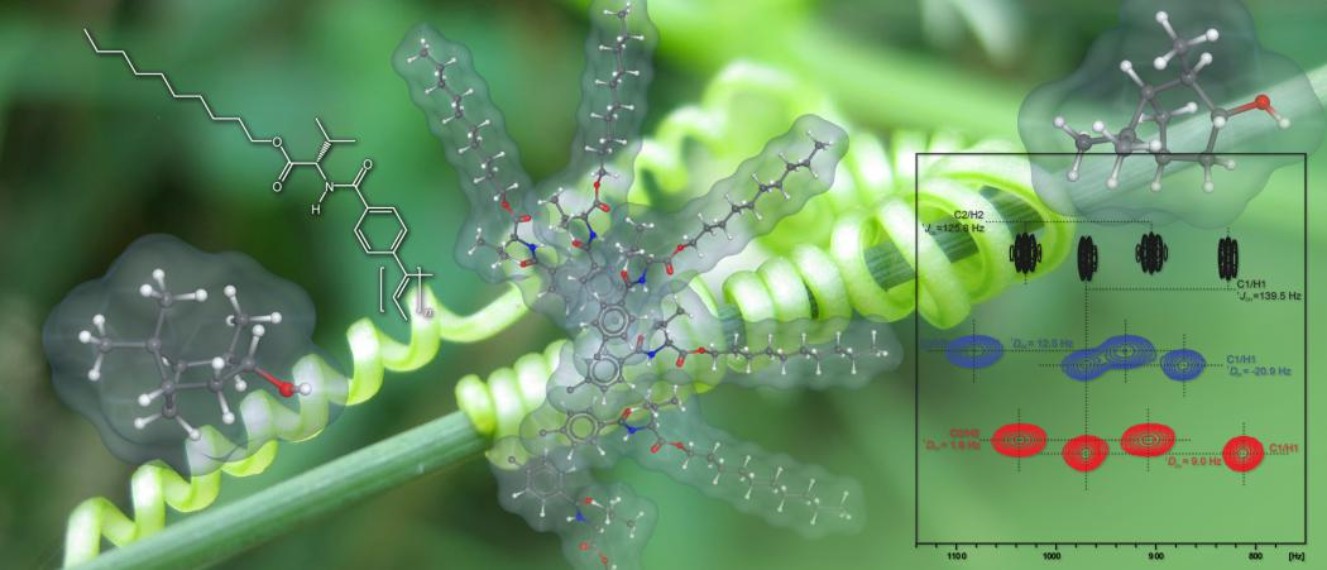
Since their discovery in the early 1950’s sulfoximines have developed to a powerful tool in organic synthesis.[Review] This is especially true for the chiral non-racemic members of this uncommonly versatile class of compounds. Early work focused on the development of asymmetric alkylidene transfer reagents and ketone resolutions based on the reversible formation of β-hydroxy-sulfoximines. Nowadays sulfoximine chemistry is characterized by a transition from stoichiometric to catalytic applications. Cyclic sulfonimidates 1 [1,2,3] have proven to be valuable intermediates for the synthesis of enantiomerically pure sulfoximines. These compounds can be elaborated further into chiral ligands for asymmetric metal catalyzed reactions[4,5] and are the basis for asymmetric allyl transfer reagents.[6,7] The latter application relies on metallated 2-alkenylsulfoximines which, after diastereoselective γ-hydroxyalkylation, can be cyclized to yield isomerically pure highly substituted N- and O-heterocyclic systems.[8,9,10,11]
After removal of the auxiliary these compounds may have significance as β-turn mimetica or can be used as building blocks in natural product synthesis. Desulfuration is possible either by reduction with Raney-nickel or samarium iodide.
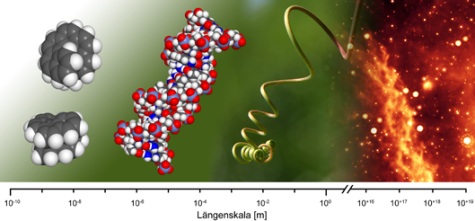
Helical objects are ubiquitous chiral objects which exist from the sub-nanometer lengths-scale up to thousands of light years. Apart from natural helical structures like nucleic acids or proteins, there exist a number of synthetic polymers with fascinating properties – known and to be discovered!
Helically chiral Polymers as Enantiodifferentiating Alignment Media
With the advent of anisotropic NMR-parameters like residual dipolar couplings (RDCs) in NMR based structure determinations, the need for media suited to hinder molecules from tumbling isotropically increased. Since several years we developed (among others) lyotropic liquid crystalline (LLC) phases of polyarylacetylenes and polyarylisonitriles for that purpose. Due to the fact that these polymers have chiral non-racemic amino acid based side chains, they are helically chiral and therefore able to orient chiral analytes in an enantioselective manner. The ultimate goal of our research in this field is the determination of absolute configurations of dissolved molecules based on this differential oder effect. This work was a project of the DFG research group FOR 934 and was continued in international cooperations with China (Deutsch-Chinesisches Zentrum: GZ 1289) and with Brasil (DFG-CAPES). Moreover, the synthetic and NMR work was extended by the development of a new computer program (ConArch+) for the rapid determination of relative configurations based on Distance Geometry calculations using all kind of NMR data including anisotropic parameters.
Helically chiral Polymers as Catalysts
In this project we pursue the idea to combine polymer-related process advantages (easy separation of polymer and products, recyclability, membrane reactor design) with chemical function. The latter not only means catalysis of a given reaction, it also implies the control of newly created stereogenic elements in the products, thus exploiting the uniform helicity of the polymer. In the early days of this research we worked with polyacrylates and polyisocyanates. Later we explored the potential of polyquinoxalines and today we focus on polyarylacetylens and polyarylisonitriles.
Organic Light Emitting Diodes (OLEDs)

Since quite a number of years the display market is changing. The still dominating LCD displays get more and more superseded by Organic Light Emitting Diodes (OLEDs). Compared to LCDs OLEDs have a number of attractive properties. They can be produced in large area, the devices may be extremely thin and flexible and they are characterized by a low energy consumption. High performance OLEDs are multi-layer devices which are usually assembled by vacuum sublimation which has a number of disadvantages that may be overcome if it would be possible to fabricate the device from solution. Apart from using orthogonal solvents this may be done by crosslinking the n-th layer before adding the next one. In collaboration with Prof. Meerholz (University of Cologne) we are working on crosslinkable molecules for all layers needed (hole transport-, electron transport- and emissive layer).
Sulfoximine Chemistry
Chiral, enantiomerically pure sulfoximines are valuable building blocks for asymmetric syntheses. Based on 2-alkenyl sulfoximines we developed solutions for asymmetric d3-synthons which ultimately led to a powerful new strategy for the synthesis of highly substituted aza-heterocyclic systems. Besides these applications as chiral auxiliaries, we sucessfully developed chiral catalysts based on mono- and bissulfoximines as ligands for late transition metals, copper and in recent time yttrium. The latter system allows for an efficient asymmetric amination of olefins.