Automotive Catalysis
In more than 60 years, the concern for anthropogenic air pollution has quickly grown. According to the European Environmental Agency, transport industry is one of the main sources of air pollution in Europe [1].
Among the main pollutants from combustion engines, it can be found CO, CO2, particulate matter (PM), volatile organic compounds (VOC), nitrogen oxides (mainly NO and NO2) and NH3 [1,2]. Some of these pollutants can also lead to other pollutants (for this referred to as secondary pollutants), which can be harmful for the environment as well as for humans [1,2,3].
For these reasons, great efforts are made to reduce the tail-pipe emissions from vehicles. To do so, it is now well established the use of catalytic converters, where the harmful gases are transformed into less harmful mixtures of preferably CO2, N2 and water.
Because of the different kinds of reactions (in general, oxidations and reductions), the conversion system is characterised by different units. Depending on the combustion engine type (either Gasoline or Diesel), different systems are used.
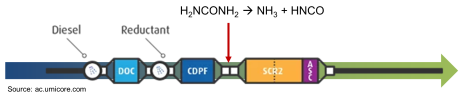
For a Diesel engine, the most up-to-date system can be represented by the following picture:
Where:
- DOC (Diesel Oxydation Catalyst) is responsible for the oxidation of C-based compounds (mainly CO and VOC) into CO2;
- DPF (Diesel Particulate Filter) is responsible for the oxidation of particulate matter (PM);
- Urea-assisted SCR (Selective Catalyitic Reduction) is responsible for the reduction of nitrogen oxides into N2 by reacting with NH3 (from injected urea);
- ASC (Ammonia Slip Catalyst) is responsible for the oxidation of excess of NH3 exiting the previous unit into N2.
One of the main challenges of this kind of catalysis – for this called Automotive Catalysis – is the extreme variability of its operative conditions, far more unpredictable than more stationary catalysis. The development of an automotive catalyst is then strongly relying on real-conditions tests, which require non-negligible costs of time and materials.
For this reason, mathematical models are now getting more and more attention, since they could provide a reliable alternative to these tests [4]. One of the challenges, in that case, is then to build reliable kinetic models which can correctly predict real-life driving conditions the catalyst will be exposed to.
Unit Layout
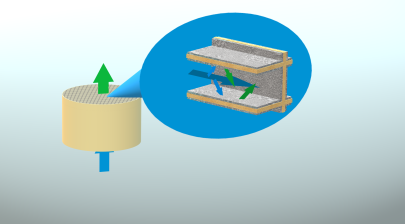
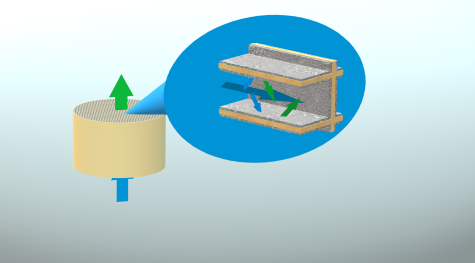
In general, each catalytic converter consists in a channelled monolith with the active material coating the walls of each channel. For this reason, such material is called washcoat. and it is a porous solid which the gas diffuses through, to be converted.
In such a system, the conversion is strongly influenced by both the chemical and morphological properties of the catalytic material, with limitations arising from both kinetics and mass transfer.
Selective Catalytic Reduction
Due to the high amount of nitrogen oxides (NOx) formed in a Diesel system, their reduction to N2 requires a dedicated unit: the SCR (Selective Catalytic Reduction) unit. In this unit, the NOx are converted by reacting with NH3, which derives from thermal decomposition of urea, injected in the stream before entering the unit. Since the main nitrogen oxides are NO and NO2, depending on their concentration in the gas mixture three main SCR mechanisms are identified:
- Standard SCR (for yNO2 ≈ 0)
4NH3 + 4NO + O2 → 4N2 + 6H2O - Fast SCR (for yNO ≈ yNO2)
4NH3 + 2NO + 2NO2 → 4N2 + 6H2O - Slow SCR (for yNO ≈ 0)
4NH3 + 3NO → 3.5N2 + 6H2O
One of the peculiarities of modern SCR catalysts is that they are metal ions supported on zeolite frameworks. Such a structure allows high selectivity and activity thanks to the significantly small porosity of zeolites (10-1 nm order of magnitude)[5]. As a consequence, the reaction mechanism of these kind of catalysts differs from the more common surface catalysis, and this is one of the reasons these technologies are still intensively studied today.
The most common SCR technology for Diesel application is Cu supported on chabazite zeolite, although alternative Fe- or V-based catalysts are also used.
Ammonia Slip Catalyst
Ammonia from urea is commonly used as reducing agent for the Selective Catalytic Reduction (SCR) of NOx. Excess ammonia has to be removed from the exhaust stream due to emission regulations because of its toxicity. This is done by an Ammonia Slip Catalyst (ASC), usually a dual layer system consisting of an Ammonia Oxidation Catalyst (AOC) and an SCR catalyst coated onto a monolithic substrate. The SCR layer is necessary to reduce NOx produced on the AOC layer (unselective reaction to N2 and NOx) and acts also as a diffusion barrier. Key research elements of the catalytic system are the NH3 conversion, N2 selectivity and the diffusion limitation in the SCR layer.
Modelling
Next to tests at synthetic gas benches nowadays the performance and behaviour of catalysts is simulated with models which reduces testing costs and time. There are different forms of models one of which are mechanistic models that solve the chemistry behind the catalyst for the applied conditions. The idea behind mechanistic models is to go beyond the calibrated limits and predict catalyst behaviour even outside of this range. We use a MATLAB model (including FORTRAN code) to simulate all types of monolithic catalysts in the exhaust aftertreatment.
Diesel Oxidation Catalyst
The increasing scarcity of fossil fuels and the goal of CO2 neutral propulsion techniques demand new energy concepts within the field of automobility. The utilization of alternative fuels based on renewable energies constitutes one possible route to achieve this objective. As a suitable additive or substitute for diesel fuel, oxymethylene ethers (OME) have gained increasing attention. They can be produced using H2 from renewable energy sources, CO2 and water, thus enabling a closed CO2 loop. Furthermore, due to its molecular structure, the combustion of OME leads to significant reductions in soot emissions, allowing for more efficient inner-engine NOx reduction methods (e.g. higher exhaust gas recirculation rates).
Using blends of Diesel and OME however poses new demands for the diesel emission control systems downstream of the engine. The key component of such a system is a Diesel Oxidation Catalyst (DOC). Besides the efficient removal of CO and hydrocarbons from the diesel engine exhaust, its further purpose is to generate heat and to produce NO2, which allows for optimal operation of downstream components such as the Diesel Particular Filter (DPF), NH3 Selective Catalytic Reduction (SCR) catalyst or Lean NOx Trap.
In a fully automated test rig, a synthetic exhaust gas is generated and passed through an DOC. Using different measuring techniques (e.g. FTIR, FID), the exhaust gas downstream of the catalyst is analyzed enabling to evaluate the catalyst performance under different exhaust gas conditions (composition and temperature). The test rig can be used for stationary measurements but also allows dynamic experiments to simulate real time driving cycles. The experimental investigations are assisted by numerical models, providing a deeper understanding of the underlying physical and chemical processes controlling the complex behavior of a DOC.
Literature
[1] European Environment Agency (EEA). Air quality in Europe -2019 report 1994-2019 EEA Report No 10/2019, 10,2019, p.104
[2] Suarez Bertoa et al., Atmosphere 11 2 (2020), 1-18
[3] Sutton et al., Ammonia in the environment: From ancient times to the present (2008)
[4] Bendrich et al., Applied Catal. B: Environ 222 (2018), 76-87
[5] Chen et al., J. Catal 329 (2015), 490-498