Following the cluster production, either charged or neutral particles can be investigated. In the latter case, charged species must be deflected and the neutral clusters ionized (Xe flash lamp or F2 excimer laser).
Absorption spectra are usually obtained by attenuating the intensity of radiation as it passes through a sample under investigation. If optical properties of clusters within a high vacuum molecular beam apparatus, are to be investigated, a different measuring method must be used. However, the particle number density in such molecular beams is too low to detect differences in radiation intensities. Absorption spectra can thus be obtained indirectly via the photodissociation of the clusters (action spectroscopy).
As a result of the laser absorption, the cluster can dissociate which is implemented instrumentally by an aperture and corresponds to a depletion of the detected ion mass signal. The measurement principle for recording absorption spectra is based on the mass spectrometric tracking of the wavelength-dependent optical response of the clusters. The central quantity for the experiment, the quantum-chemically interpretable absorption cross section, is then obtained from a modified Beer-Lambert law.
The overlap parameter α between the laser and the molecular beam as well as the photon fluence ϕ as a measure for the laser intensity must be determined in separate experiments.
The ionization potential is the energy required to remove an electron from the highest occupied molecular orbital (HOMO).
If the energy is introduced into the system via light irradiation, the process is called photoionization, which is shown schematically in Fig. 2.
When detecting photoionization using a time of flight (ToF) mass spectrometer, it is important to note that only charged particles can be detected.
If the energy of the monochromatic light is now gradually increased, an increase in cluster intensity can be observed when the ionization potential is reached.
In this experiment the monochromatic light is generated by a laser system. To reach the energy regions required for most cluster systems, the so called wavelength mixing is used, which requires multiple lasers and mixing mediums.
The comparability of redox potential and ionization potential becomes visible when looking at the oxidation shown in Fig. 3.
Thus a photoionization can be regarded as oxidation by light, whereby the redox potential can be inferred from the ionization potential.
When comparing experimental spectra and theoretical predictions from time-dependent quantum chemistry (cf. Fig. 4), the photodissociation spectroscopy can be used as a structure and spin state elucidating tool.
Prior to calculating absorption spectra, the potential structural candidates of the clusters need to be found. In this comtext a Genetic Algorithm based on evolutionary principles and coupled to density functional theory (DFT) has been proven satisfactory to generate geometries representing local minima on the potential energy surface. The following time-dependent quantum chemistry can either be applied either on the DFT level of theory as well or using more precise ab initio methods such as Coupled Cluster and multiconfigurational approaches.
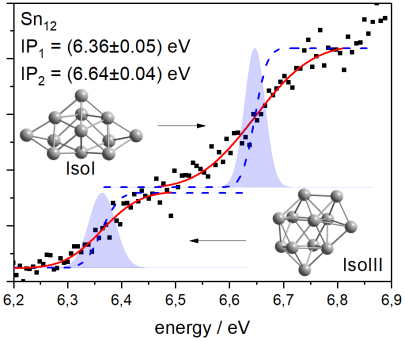
The geometries obtained by the Genetic Algorithm can also be used for the calculation of so-called photoelectron spectra. These photoelectron spectra are the derivatives of the photoionization curves measured during ionization spectroscopy. Thus, by integrating the calculated photoelectron spectra, a theoretical photoionization curve can be calculated. This can then be compared with the experimental curve to draw conclusions about, for example, the structure of the clusters (Fig. 5).
Literature
A. Shayeghi, R. L. Johnston, R. Schäfer, Phys. Chem. Chem. Phys. 2013, 15, 19715.
M. Jäger, Dissertation, TU Darmstadt, 2021.
M. Jäger, A. Shayeghi, V. Klippenstein, R. L. Johnston, R. Schäfer, J. Chem. Phys. 2018, 149, 244308.
A. Lehr, M. Jäger, R. Schäfer, J. Phys. Chem. C 2020, 124, 1070-1076.
S. Gozem and A. I. Krylov, WIREs Comput. Mol. Sci, 2022, 12, 551.