116
L. Laux, C. M. Thiele
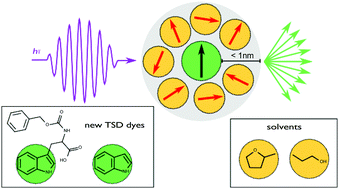
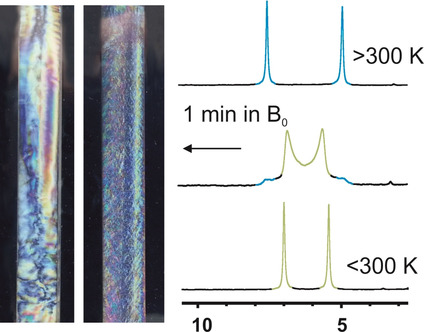
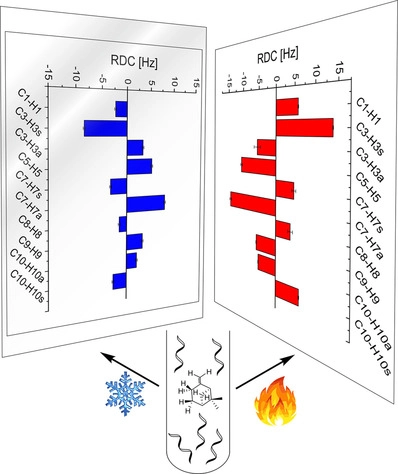
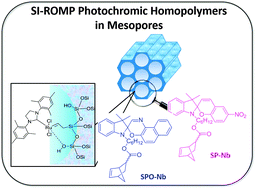
Sweet Sidechain: A Glycopeptide-based Alignment Medium to Measure Residual Dipolar Couplings in Neat Acetonitrile
J. Mater. Chem. C, 2025, 13, 19848-19855
DOI: 10.1039/D5TC02303C
[raw data DOI: 10.5281/zenodo.16628414]
115
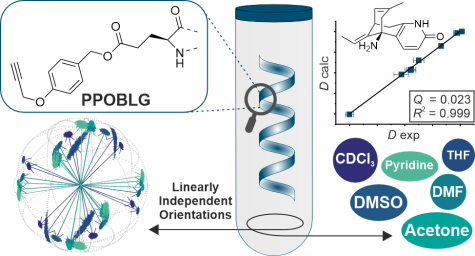
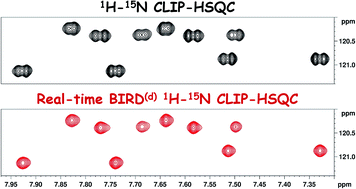
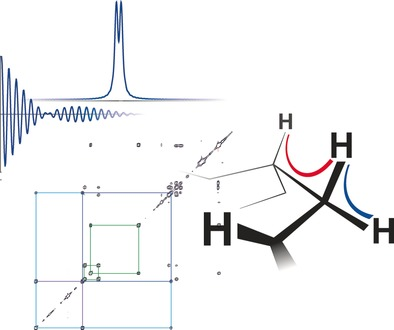
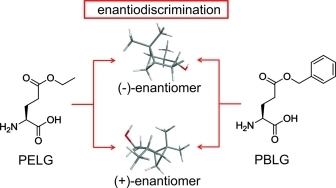
L. Laux, M. Alcaraz Janßen, C. M. Thiele
A Highly Versatile Enantiodifferentiating Polyglutamate Alignment Medium to Measure Residual Dipolar Couplings in non-Polar and Polar Solvents
J. Am. Chem. Soc., 2025, 147, 33634-33642
DOI: 10.1021/jacs.5c09152
[raw data DOI: 10.5281/zenodo.16738060]
114
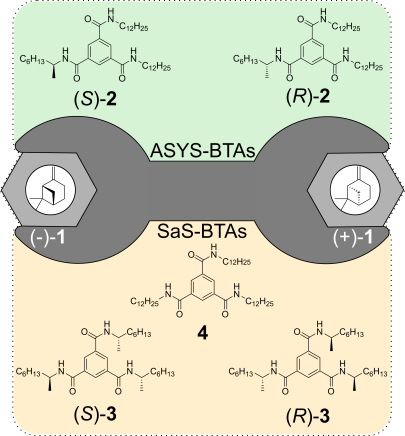
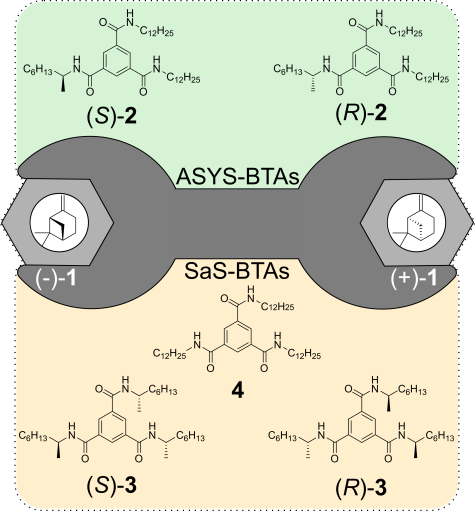
M. Brauser, M. Leyendecker, V. Schmidts, C. M. Thiele
Spin Me Right Round – A Versatile BTA-Based Alignment Media Toolbox for Enhanced Enantiodiscrimination
Chem. Commun., 2025, 65, 12729-12732
DOI: 10.1039/D5CC02116B
113
J. S. Doll, J. Kergassner, B. Zhang, C. M. Thiele, G. Buntkowsky, M. Enders, T. Gutmann, D.-A. Roşca
Highly active iron catalysts for olefin hydrogenation enable parahydrogen induced hyperpolarisation of 1H and 19F NMR resonances at 1.4 Tesla
Chem. Commun., 2025, 61, 11421-11424.
DOI: 10.1039/D5CC02409A
112
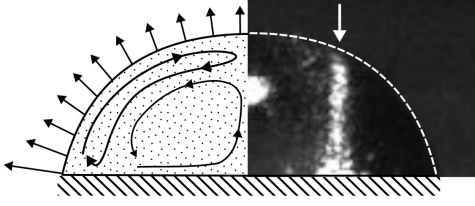
A. Erb, J. Kind, T.L. Zankel, R.W. Stark, C. M. Thiele
Visualization and quantification of local concentration gradients in evaporating water/glycerol droplets with micrometer resolution
Proc. Natl. Acad. Sci. U.S.A. 2025, 122, (20), e2423660122.
[Raw data DOI: 10.48328/tudatalib-1498]
111
J. Rettig, M. Gölz, C. M. Thiele
Synthesis and Application of a Hydrophobic Polyglutamate Bearing a Triphenylphosphine Group for the Orientation of Pharmaceutically Active Compounds and the Measurement of Residual Dipolar Couplings
Magn. Reson. Chem. 2025, 63, 406-413.
DOI: 10.1002/mrc.5522
[Raw data DOI: 10.5281/zenodo.14170603]
110
M. Brauser, K. Petzold, C. M. Thiele
Investigating Interaction Dynamics of an Enantioselective Peptide-Catalyzed Acylation Reaction
Angew. Chem. Int. Ed. 2025, 64, e202421062.
[Raw data DOI: 10.5281/zenodo.13983993]
109
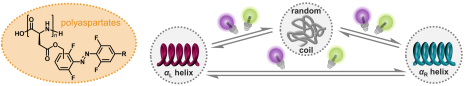
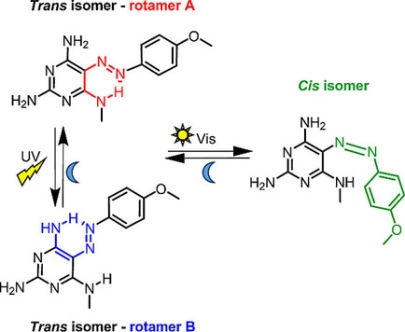
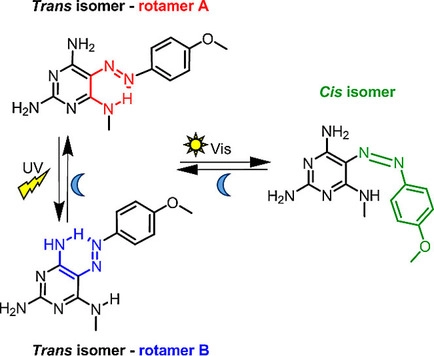
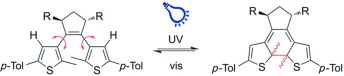
R. Hossain, C. M. Thiele
Exciting Novel Polyaspartates: Design, Synthesis, and Photo-Responsive Behavior in Solution and Lyotropic Liquid Crystalline Phase Upon Irradiation with Visible Light
Macromol. Rapid Commun. 2024, 45, 2400513.
108
F. Trauner, R. Ghazali, J. Rettig, C. M. Thiele, D. Didier*
Stereoselective polar radical crossover for the functionalization of strained-ring systems
Commun. Chem. 2024, 7, 139.
DOI: 10.1038/s42004-024-01221-3
[Supplementary Data DOI: 10.6084/m9.figshare.25907506]
107
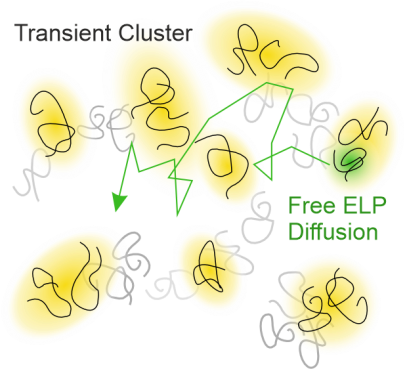
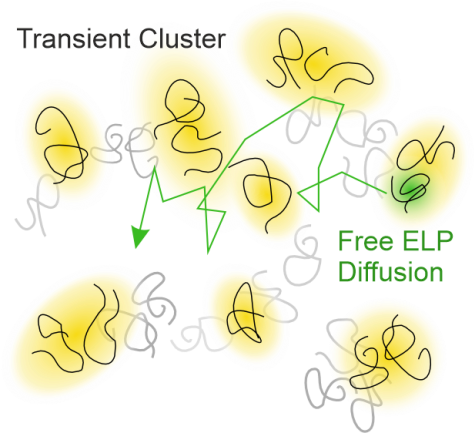
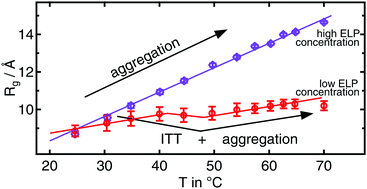
S. Weißheit, B. Kuttich, M. Vogel, C. M. Thiele
Elastin-like Peptide as a Model for Disordered Proteins: Diffusion Behaviour in Self-Crowding Conditions
ChemPhysChem 2024, 25, e202400117.
106
Editors: L. Yao, B. Vogeli
Residual Dipolar Couplings: Principles and Applications
J. Rettig, M. Brauser, C. M. Thiele
Chapter 11: Structure Determination of Organic Molecules Using RDCs (and RCSAs)
RSC 2024, ISBN: 978-1-83916-429-3.
105
D. Herold, M. Brauser, J. Kind, C. M. Thiele
Evolution of a Combined UV/Vis and NMR Setup with Fixed Pathlengths for Mass-limited Samples
Chem. Eur. J. 2024, 30, e202304016.
104
J. Nowag, M. Brauser, L. Steuernagel, R. C. Wende, P. R. Schreiner, C. M. Thiele
Quantifying Intermolecular Interactions in Asymmetric Peptide Organocatalysis as a Key toward Understanding Selectivity
J. Am. Chem.Soc. 2024, 146, 170–180.
DOI: 10.1021/jacs.3c06378
[Raw data DOI: 10.5281/zenodo.7872628]
103
D. Herold, J. Kind, F. Frieß, C. M. Thiele
Extraction of pure component spectra from ex situ illumination UV/Vis and NMR spectroscopy
Photochem. Photobiol. Sci. 2023, 22, 2599–2606.
102
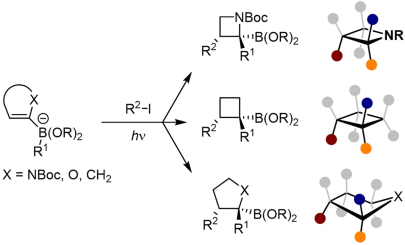
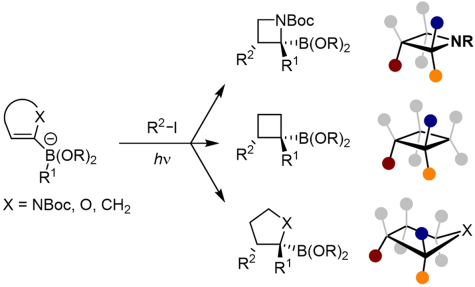
J. J. Primozic, J. Ilgen, P. Maibach, M. Brauser, J. Kind, C. M. Thiele*
Pd-Catalyzed Asymmetric Allylic Alkylation of Cyclobutenes: From Double Inversion to Double Retention
J. Am. Chem. Soc. 2023, 145, 15912-15923.
DOI: 10.1021/jacs.3c03590
101
F. Theiss, L. Wienands, J. Lins, M. Alcaraz Janßen, C. M. Thiele, G. Buntkowsky
Parahydrogen-induced polarization enables the single-scan NMR detection of a 236 kDa biopolymer at nanomolar concentrations
Sci. Rep. 2023, 13, 10117.
100
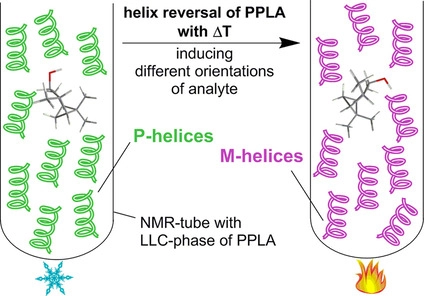
T. Imhof, J. Kind, C. M. Thiele
Tilting or Twisting? Using Anisotropic Solvent Diffusion in Polymeric Thermo-Responsive LLC Phases for the Distinction of Spatial Reorientation versus Inversion of the Helical Polymer Backbone
Macromolecules 2023, 56, 2947-2954.
DOI: 10.1021/acs.macromol.2c02021
[Raw data DOI: 10.23728/b2share.7e9406cbc86549cbb16e221957e15edb]
99
M. Hirschmann, O. Soltwedel, P. Ritzert, R. von Klitzing, C. M. Thiele
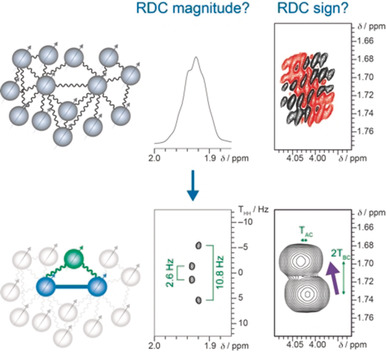
Light-Controlled Lyotropic Liquid Crystallinity of Polyaspartates Exploited as Photo-Switchable Alignment Medium
J. Am. Chem. Soc. 2023, 145, 3615-3623.
DOI: 10.1021/jacs.2c12760
98
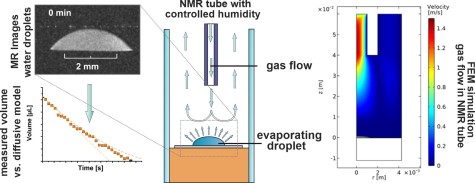
J. Kind, M. Stein, T. Gambaryan-Roisman, P. Stephan, T. L. Zankel, C. M. Thiele
Construction of an active humidity regulation setup for NMR/MRI-Observation and simulation of the controlled evaporation of sessile water droplets
J. Magn. Reson. 2023, 348, 107389.
DOI: 10.1016/j.jmr.2023.107389
[Raw data DOI: 10.23728/b2share.a58444ebaf8b4cbeba0c3d4237c58dc3]
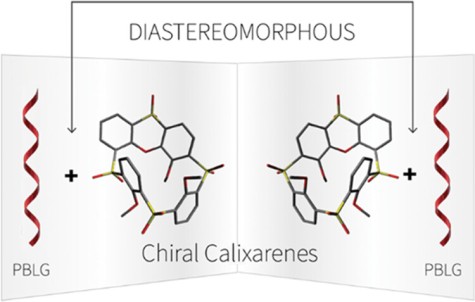
97
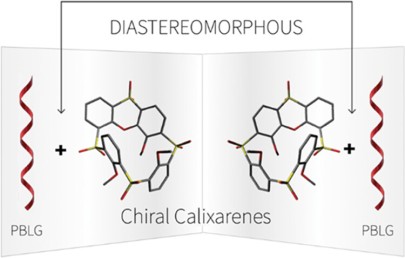
M. Tichotová, T. Landovský, J. Lang, S. Jeziorowski, V. Schmidts, M. Kohout, M. Babor, P. Lhoták,* C. M. Thiele,* H. Dvořáková*
Enantiodiscrimination of Inherently Chiral Thiacalixarenes by Residual Dipolar Couplings
J. Org. Chem. 2024, 89, 9711-9720.
96
M. Brauser, T. Heymann, C. M. Thiele
Detection and Verification of a Key Intermediate in an Enantioselective Peptide Catalyzed Acylation Reaction
Molecules 2022, 27, 6351.
95
P. Weigl, S. Weißheit, F. Pabst, H. Kolmar, C. M. Thiele, T. Walther, T. Blochowicz
Triplet States Reveal Slow Local Dynamics in the Solvation Shell of Biomolecules
J. Phys. Chem. B 2022, 126, 6324-6330.
94
D. S. Schirra, P. Götz, M. Lehmann, C. M. Thiele
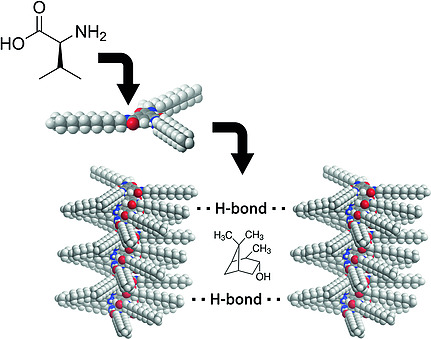
Atropisomerism in a polyglutamate-based thermoresponsive alignment medium
Chem. Commun. 2022, 58, 7511-7514.
DOI: 10.1039/D2CC01982E
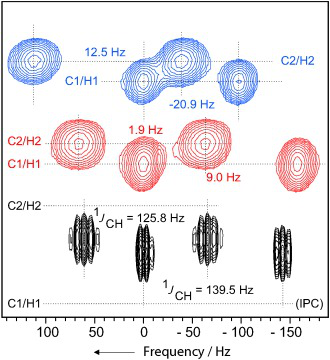
93
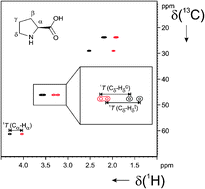
D. S. Schirra, S. Jeziorowski, M. Lehmann, C. M. Thiele
Thermoreversible Gelation of Homopolyglutamates PBPMLG, PBPELG, and PBPHLG: Another Step toward de Novo RDC-Based Structure Elucidation
Macromolecules 2022, 55, 3430-3436.
DOI: 10.1021/acs.macromol.2c00176
[Correction: 10.1021/acs.macromol.2c01242]
92
A. K. Bell, J. Kind, M. Hartmann, B. Kresse, M. V. Höfler, B. B. Straub, G. K. Auernhammer, M. Vogel, C. M. Thiele, R. W. Stark
Concentration gradients in evaporating binary droplets probed by spatially resolved Raman and NMR spectroscopy
PNAS 2022, 119, e2111989119.
[Raw data DOI: 10.48328/tudatalib-824.2]
91
K. Knoll, D. Herold, M. Hirschmann, C. M. Thiele
A Supramolecular and Liquid Crystalline water-based Alignment Medium based on Azobenzene-substituted 1,3,5-Benzenetricarboxamides
Magn. Reson. Chem. 2022, 60, 563-571.
DOI: 10.1002/mrc.5266
[Raw data DOI: 10.5281/zenodo.6020398]
90
K. Knoll, T. Kostner, C. Lorenz, C. M. Thiele
Investigations into Supramolecular Lyotropic Liquid Crystals based on 1,3,5-Benzenetricarboxaramides by NMR-spectroscopy
Eur. J. Org. Chem. 2022, 22, e202101490.
89
F. A. Roth, V. Schmidts, J. Rettig, C. M. Thiele
Model Free Analysis of Experimental Residual Dipolar Couplings in Small Organic Compounds
Phys. Chem. Chem. Phys. 2022, 24, 281-286
DOI: 10.1039/D1CP02324A
[Raw Data: 10.5281/zenodo.5636052, Pre-Print: 10.26434/chemrxiv.14636316.v1]
88
F. A. Roth, V. Schmidts, C. M. Thiele
TITANIA: Model-Free Interpretation of Residual Dipolar Couplings in the Context of Organic Compounds
J. Org. Chem. 2021, 86, 15387-15402
[Source Code: DOI 10.5281/zenodo.5548105, Raw Data: DOI 10.5281/zenodo.5556977, Pre-Print: DOI 10.26434/chemrxiv.14636070.v1]
87
D. S. Schirra, M. Hirschmann, I. A. Radulov, M. Lehmann, C. M. Thiele*
Investigations of the alignment process of homopolyglutamate-based LLC phases: Deuterium NMR analysis of PBPMLG reveals a 90° flip of the polymer
Angew. Chem. 2021, 133, 21208-21214, Angew. Chem. Int. Ed. 2021, 60, 21040-21046.
86
M. Hirschmann, D. S. Schirra, C. M. Thiele*
Copolyaspartates: Uncovering Simultaneous Thermo and Magnetoresponsiveness
Macromolecules 2021, 54, 1648-1656
85
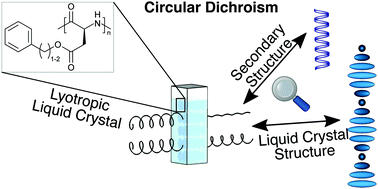
M. Hirschmann, C. Merten, C. M. Thiele*
Treating Anisotropic Artefacts in Circular Dichroism Spectroscopy Enables Investigation of Lyotropic Liquid Crystalline Polyaspartate Solutions
Soft Matter 2021, 17, 2849-2856
DOI: 10.1039/D0SM02102D
84
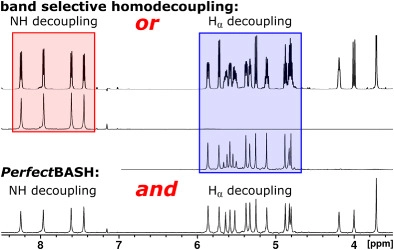
J. Ilgen, J. Nowag, L. Kaltschnee, V. Schmidts, C. M. Thiele*
Gradient selected pure-shift EASY-ROESY techniques facilitate the quantitative measurement of 1H,1H-distance restraints in congested spectral regions
J. Magn. Reson. 2021, 324, 106900
DOI: 10.1016/j.jmr.2020.106900
Preprint: PDF (wird in neuem Tab geöffnet) | SI (wird in neuem Tab geöffnet)
83
P. Weigl,* D. Schadt, S. Weißheit, C. M. Thiele, T. Walther, T. Blochowicz
Triplet state solvation dynamics: extending the accessible timescale by using indole as local probe
Phys. Chem. Chem. Phys. 2021, 23, 683-693
DOI: 10.1039/D0CP05240J