A refreshing approach to probing complex reaction pathways
2020/07/27 by B.J.M. Etzold
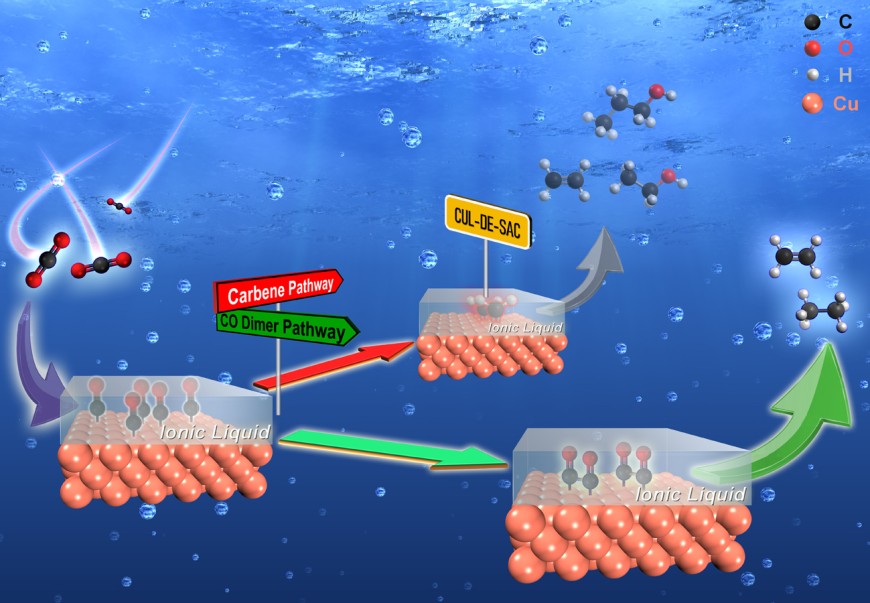
With the aim to use renewable energy directly for driving chemical conversion processes, gaining a mechanistic understanding of electrocatalytic processes is a major scientific question studied nowadays. A well-known case is the electrochemical CO2 reduction on copper catalysts. This reaction would allow to convert CO2 e.g. from recycling streams within the circular economy or stemming from industrial off gases or CO2 captured from the atmosphere to be converted to the building blocks of various chemical products. Due to the involvement of multiple H+/e- transfers and the associated complex reaction network, gaining mechanistic insights into this reaction is highly challenging. Major techniques to obtain mechanistic insights rely heavily on instrument intensive spectroscopic approaches. Recently, a research group led by Prof. Bastian Etzold at the Ernst-Berl-Institut für Technische und Makromolekulare Chemie has proposed a new facile way to disentangle the complex reaction networks of CO2 reduction on Cu catalysts by employing ionic liquid as a chemical trapping agent, which is recently published in the renowned journal Angewandte Chemie International Edition (DOI: 10.1002/anie.202009498).
Previous works
The idea of introducing ionic liquid into a solid catalyst can date back to 13 years ago, when Prof. Etzold was one of the pioneers in proposing the “solid catalyst with ionic liquid layer (SCILL)” concept for modulating a heterogeneous catalyst (Ni/SiO2) for a selective hydrogenation reaction (Chem. Eng. Technol. 2007, 30, 985-994). Inspired by the great success of the SCILL concept in thermal catalysis, Etzoldlab transferred this concept into electrocatalysis and demonstrated that the presence of a subtle amount of ionic liquid can dramatically boost the activity of carbon supported Pt catalyst for the oxygen reduction reaction, which represents a major bottleneck for the low temperature fuel cell technology. Within the running ERC Consolidator Grant IL-E-Cat, the influences of the chemical identity of catalysts, loading amount and molecular variation of ionic liquid have been comprehensively studied, and the role of ionic liquid in preserving the active sites for the oxygen reduction was explicitly identified for the first time. The success of these pioneering works leads to several inspiring publications (ACS Appl. Mater. Interfaces 2015, 7, 3562-3570; Angew. Chem., Int. Ed. 2016, 55, 2257-2261; J. Power Sources 2018, 375, 222-232; ACS Catal. 2018, 8, 8244-8254; ACS Catal. 2019, 9, 8682-8692) and also establishes a leading position of Etzoldlab in applying the SCILL concept in electrocatalysis. The transfer of the SCILL concept towards the electrochemical CO2 reduction leverages this backdrop and is part of the ERC Consolidator Grant IL-E-Cat.
A unique perspective to track CO2 reduction pathways
Electrochemical CO2 reduction is expected to play a key role in enabling a sustainable solar power-based economy, while mechanistic understanding of the reaction pathways, which provides the basis of steering the CO2 reduction toward desired products, remains controversial. In view of this, the electrochemistry research team at the lab of Prof. Bastian J.M. Etzold (Chemistry, TU Darmstadt), in collaboration with Prof. Jan P. Hofmann (Surface Science Laboratory, TU Darmstadt) and Dr. Ioannis Katsounaros (Helmholtz Institute Erlangen-Nürnberg for Renewable Energy), has proposed a new and easy-to-implement approach to gaining electrochemical reaction mechanisms of the copper catalyzed CO2 reduction. In their work, an ionic liquid was immobilized on the catalyst and it could be shown that the ionic liquid acts as a chemical trapping agent. The research showed that the ionic liquid molecules significantly altered the product spectrum by selective suppressing the formation of ethylene, ethanol, and n-propanol, likely by trapping a common key intermediate (e.g. carbene), while formation of other products is more or less undisturbed. The response in product distribution to the ionic liquid modification demonstrates a unique way to simplify the complex reaction networks of various products. Importantly, this unprecedented yet simple approach can be applied to existing electrochemical cells, which allows not only the application in every electrochemical laboratory, but also the use with existing specialized setups, where a spectroscopic access cannot easily be realized, but which can e.g. operate under technically relevant conditions. Considering the great variety of ionic liquids, it is believed that chemical trapping using ionic liquids has the potential to become the new generic method for deriving mechanistic insights in electrocatalysis, and paves the way to selectively electrosynthesis for desired products.
The publication:
Probing CO2 Reduction Pathways in Copper Catalysts using Ionic Liquid as a Chemical Trapping Agent, G.-R. Zhang, S.-D. Straub, L.-L. Shen, Y. Hermans, P. Schmatz, A.M. Reichert, J.P. Hofmann, I. Katsounaros and B.J.M. Etzold, Angew. Chem. Int. Ed., DOI: 10.1002/anie.202009498.
