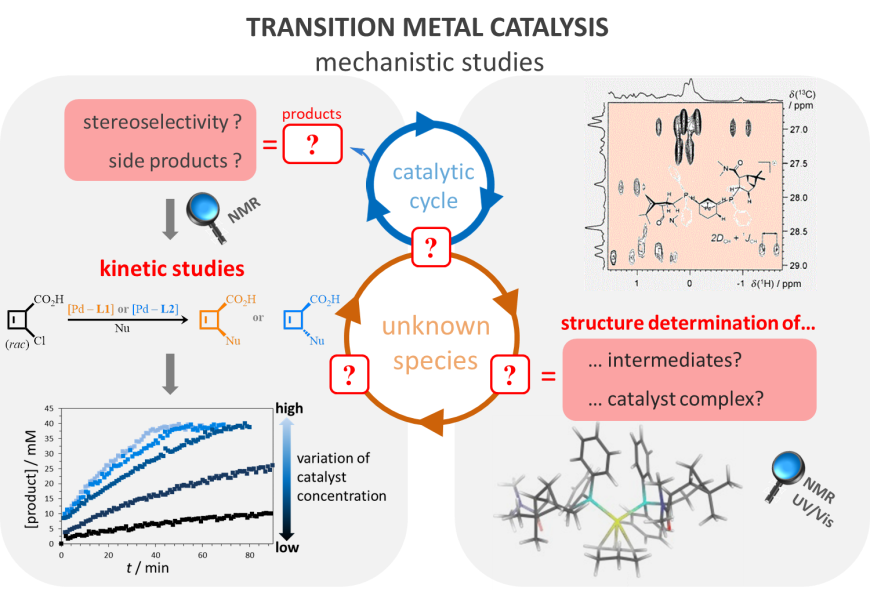
Many of our projects aim at the determination of the constitution and configuration of small organic compounds in solution by NMR spectroscopy. Using the information about the structure of the compound obtained this way, we hope to learn more about its (bio-)chemical function and better understand its reactions (structure-activity relationship). Often, a simple rigid structure model is insufficient to describe the experimental findings, so that the conformational ensemble averaging and dynamic processes have to be included in the analysis. We also investigate reaction mechanisms with unknown intermediates in organocatalysis and transition metal catalysis, dynamic processes in compounds and intermolecular interactions to contribute to a better understanding of structure-activity relationships.
To facilitate structure determination, we combine information from complementary NMR parameters, namely nuclear Overhauser enhancements (NOEs), scalar couplings (J-couplings) and residual dipolar couplings (RDCs). In cases where the J-coupling pathway is discontinuous or the NOEs do not allow for an unambiguous determination, the analysis of RDCs may clear up the assignment of diastereotopic protons, as we were able to show already in 2003 for the model compound strychnine.
We further use RDCs for the determination of the relative configuration of stereogenic centers, as in the case of the marine natural product Dibromopalau’amine, where the classical assignment by J/NOE-based methods was not entirely conclusive due to the unusual, highly substituted polycyclic scaffold. An additional challenge is present in cases of conformationally flexible compounds, where NMR parameters are only observed as averages and the inference of the contributing conformers is often impossible. The determination of the relative configuration of the α-methylene-γ-butyrolactone failed due conformational flexibility of the five-membered ring. Only the analysis of RDCs led to the unambiguous determination of the trans relative configuration of the stereogenic centers with the simultaneous description of the conformer ensemble in solution.
Currently, we are working on further structure determination projects of challenging compounds, with e.g. stereogenic centers at exocyclic positions with potentially freely rotatable single bonds. This requires a much higher number of structure models and experimental RDCs to fully sample the configurational and conformational space with sufficient precision. One of our over-arching research goals is to deal with this increase in complexity by applying the recently established model-free RDC analysis method that gives an accurate description of a compound’s structure without relying on explicitly defined structure models (de novo structure determination) also for conformationally flexible compounds.
We are also interested in the investigation of organocatalytic and transition metal-catalyzed reactions. In some cases, we relate results from different methods like NMR and UV-vis spectroscopy or use combined UV-Vis-NMR methods. Specifically, these transition metal-catalyzed reactions include iron-catalyzed cross-coupling reactions, ruthenium-catalyzed metathesis reactions and palladium-catalyzed stereoselective allylic alkylation reactions, for all of which key steps of the catalytic cycle or intermediates involved are unknown. The latter example employs specific palladium-ligand complexes to simultaneously achieve the de-racemization and de-epimerization of a substrate into products with very high diastereomeric and enantiomeric excess. The reasons for this reactivity are still unknown, which is why our research focusses on the identification of potential intermediates and the key steps of the reaction mechanism.
Apart from metal-catalyzed reactions we also investigate a peptide catalyst that exhibits a very high selectivity as an organocatalyst in the kinetic resolution of racemic cyclohexanediols. Computation suggests that the tetrapeptide assumes a conformation resembling an enzyme pocket. We were already able to find spectroscopic evidence for this conformation. Our current goal is to either find a relation of this structure with the structure of a potential intermediate or determine it in the presence of the substrate. It is of paramount interest to find out why the interaction with one of the two enantiomers is apparently much faster than with the other enantiomer. The investigation of intermolecular interactions is essential to answer this question.
The structure and dynamics in solution not only play a key role in catalytical processes but also for switchable compounds. By irradiation of photochromic compounds like azobenzenes, spiropyranes and diarylethenes with light of a characteristic wavelength, the structure and thus the properties of such compounds can be switched reversibly. The highest information content is available when irradiating these compounds inside the NMR spectrometer. We do this with our home-built in situ irradiation setup. We use these methods not only to characterize photochromic systems in more detail, but we are also interested in photocatalytic processes. We were also able to use RDCs to investigate the dynamics of a photoswitchable organocatalyst and thereby provide important contributions to the understanding of its reactivity.
These projects have inspired us to use light as a stimulus in other research projects. Currently we work on creating photoswitchable alignment media.
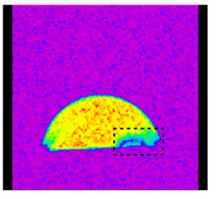
We also carry out measurements with localized NMR spectroscopy and NMR imaging. This allows us to study dynamic processes in sessile liquid droplets on a functionalized surface. We investigate the composition and droplet dynamics of binary liquid mixtures. This allows following the temporal evolution of the composition, for example, during evaporation of the droplet.