About
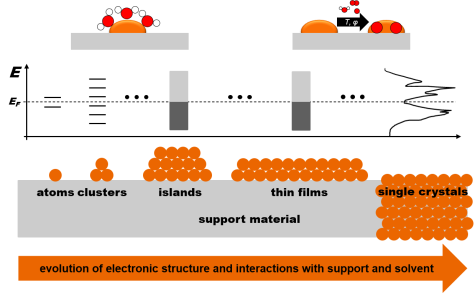
The electronic properties of iron such as the band structure, work function, ionization potential and electron affinity, which determine its redox properties in solid state and liquid phase reactions, sensitively depend on the chemical, coordinating environment of the Fe species in question. Employing lab- and synchrotron-based photoemission and X-ray absorption spectroscopies together with complementary surface science methods, a comprehensive picture of the influence of the chemical environment on the electronic configuration in both valence band and conduction band regions of well-defined model systems including Fe atoms, ionic species, cluster, thin films, bulk will be derived. Interaction with water, oxygen and hydrogen under gas phase and electrochemical conditions will be studied and dependencies on Fe species size, morphology and nature of the support with redox properties will be systematically investigated in tight interplay with theoretical modelling.
Research Team
| Name | Contact | |
|---|---|---|

| Prof. Dr. Jan Philipp Hofmann | hofmann@surface.tu-... +49 6151 16-20779 L2|02 C 406 |

| Sun Myung Kim PhD student | skim@surface.tu-... +49 6151 16-24777 L2|02 C404 |