Iron, upgraded!
Iron is a versatile, ubiquitous, cheap and sustainable transition metal with unique properties and high reactivity. Changes in the local environment modulate the intrinsic properties. In most environments, a broad variety of oxidation and spin states is easily accessible. On one hand, this can result in unfavourable or undesirable properties, on the other hand a change in the environment can be used to improve the reactivity in a targeted manner. The CRC initiative Iron, upgraded! aims at manipulating iron’s environment in such a way that it becomes a selective, versatile and valuable substitute for rare, toxic or critical metals. Three phases are envisioned: (1) Understand Iron, (2) Tune Iron, (3) Optimize Iron. In the first four years, the basic properties of iron and their dependence on the environment will be explored. In the second phase, strategies for a targeted tuning of the coordination sphere will be developed and tested. In the third phase, iron will be optimized with respect to specific properties and an improved stability, selectivity or reactivity. We further aim at transferring new or improved concepts to other 3d-elements.



Thematic Orientation
Iron will be explored in three different environments, oxidic, (pseudo-)molecular and metallic, to identify the respective inherent properties. We use magnetism, different spectroscopies and catalytic reactions to probe and understand the influence of the environment. This broad perspective coupled with an overarching interpretation allows us to draw application-specific and general conclusions. We envision that this approach enables a future use of iron in a broad variety of applications where iron and its compounds will take over diverse roles and functions: as catalyst, redox or structural promotor, in magnetic or functional materials. To elucidate the interplay of redox properties, selectivity, reactivity and stability, the use of specific and precise analytical methods is mandatory. We will therefore apply and further develop unique and coupled in situ/operando methods and new theoretical approaches.
Why Now?
The aim to upgrade iron is a timely and relevant topic. Several chemical processes and materials are based on rare, expensive, toxic or environmentally harmful metals such as ruthenium, platinum, palladium or lead. There is an urgent demand for innovative and sustainable materials and processes. For example, cheap catalysts are required for the oxygen reduction reaction in fuel cells. Embedded in the right environment, iron is an ideal candidate for this task as well as for several other applications. The CRC Iron, upgraded! conducts basic research as a foundation to realise this vision.
A – Oxidische Umgebungen
A01: Ab initio modeling of Fe in molecular environments
A02: Structural dynamics of iron molybdate catalysts
A03: Activity-stability-transport correlations of iron molybdate-based catalysts
A04: Intermetallic iron compounds and complex oxides
A05: Oxoferrates and iron oxide fluorides of higher oxidation steps
B – (Pseudo)Molecular Environments
B01: Prediction of redox potentials
B02: Properties of extended macrocylic FeN4 complexes
B03: NMR spectroscopy of open-shell iron compounds
B04: Magnetic interactions in iron dimers
B05: Operando-NMR und UV/Vis-Monitoring
B06: FeN4 centers in 3D-CNTs
B07: In situ multi-frequency EPR
B08: FeN4 interactions with clusters and nanoparticles.
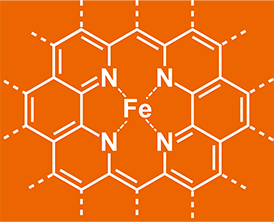
C – Metallic environments
C01: Tailored magnetic properties through interstitial defects
C02: Magnetism in Fe-based intermetallic compounds
C03: Tetragonalization of Fe-based solid alloys
C04: In situ NMR spectroscopy of iron-catalyzed reactions
C05: Selective alkynhydrogenation
C06: Influence of coordination environment on electrochemical stability
C07: Influence of tetrelatoms as ligands
C08: Electronic structure and redox properties of iron



