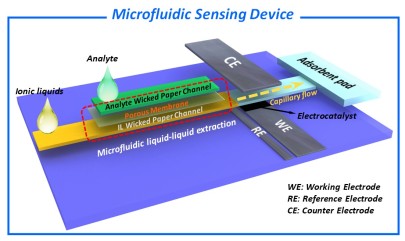
Immobilization of ionic liquids on microfluidic paper as a basis for electrochemical sensors
The Goal of the project is the development of functional devices based on ionic liquids (IL) and microfluidic paper substrates to provide a novel platform for electrochemical sensor applications. Such devices can exhibit a large potential to overcome major drawbacks of existing paper-based systems using aqueous electrolytes (e.g. low electrochemical potential windows, undesirable evaporation effects and measurement inaccuracies derived thereof). Furthermore, the solvent properties of IL can be utilized within the device for selective dissolution of different analytes.
The project will focus on the development of a sensor device for qualitative as well as quantitative detection of heavy metal ions. Since heavy metals are non-biodegradable, environment pollution by such materials is a critical issue, in particular in developing countries. Currently, the standard procedures for detection and analysis of this environmental pollution require complex analytical processes, as well as experimental skills. Consequently, simple methods for analysis which can be carried out with low instrumental effort, such as paper-based electrochemical sensors, if successfully developed, offer great potential.
Paper-intrinsic (e.g. porosity, fiber-type or pre-treatment), as well as fiber surface functionalization, have been used in the recent past to tailor the microfluidic flow of aqueous phases inside paper-based devices. For targeted utilization of ionic liquids as the mobile phase, however, several fundamental scientific questions concerning the interaction of the IL with the paper substrate (i), the electrocatalyst (ii) and the analyte (iii), are still challenging: Which paper-intrinsic and -extrinsic parameters do have an influence on fluid dynamics of IL in the porous matrix of paper substrates? Can the capillary flow of IL in the paper be controlled by rational design of the microfluidic platform? Which promoting/limiting/poisoning properties of IL affect the electrochemical detection? Can the solution or diffusion behavior be utilized for selective extraction and for increase of selectivity in multicomponent analysis?
Through a distinct set of generic experiments on the interaction of IL with the paper substrate, the electrocatalysis and the role of the analyte, fundamental mechanisms will be explored, which serves as base to answer the above outlined major scientific questions. With respect to the latter, the expertise of Prof. Etzold and Prof. Biesalski perfectly complement each other by combining experience in generation and investigation of microfluidic paper with expertise on the behavior of IL in porous media and expertise on electrocatalytically active materials.