Molecular mapping of the immunophilin-glucocorticoid-receptor complex reveals key step in the human stress response
2023/11/10 by F. Hausch
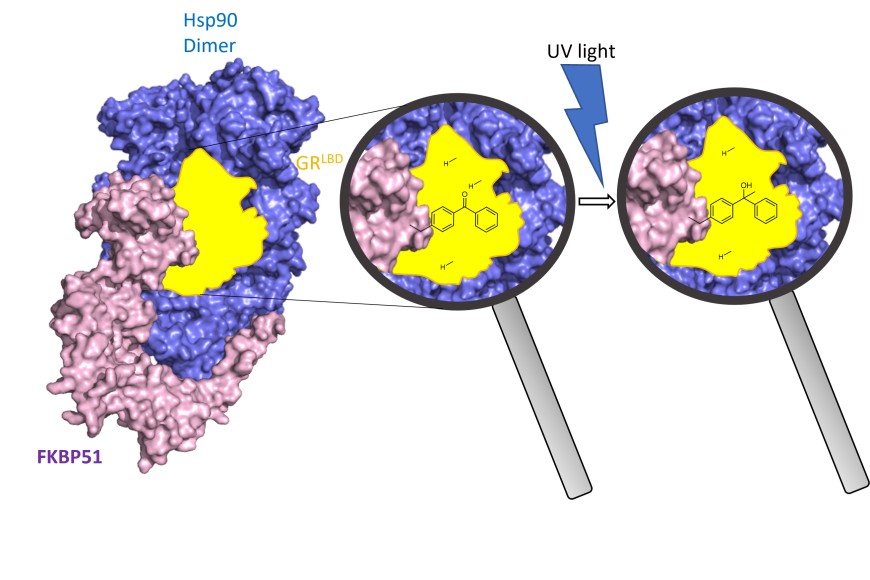
Stress signals and hormone stimuli are processed by steroid hormone receptors that are controlled by the regulatory proteins FKBP51 and FKBP52. These proteins play key roles in stress-related disorders and correct embryonic development, respectively. How FKBP51 and FKBP52 act on steroid hormone receptors is unknown.
In a recent publication in in the prestigious journal Nature Structural and Molecular Biology, Felix Hausch and coworkers elucidated the architecture and functional mechanism of FKBP51 and FKBP52 in complex with the glucocorticoid receptor and the chaperone Hsp90. ‘By systematic incorporation of photoreactive amino acids inside human cells we were able for the first time to map the intimate contacts of FKBP51 and FKBP52 with the glucocorticoid receptor inside living human cells’, explains Asat Baischew, first author of the publication. ‘This allowed us to reconstruct a snapshot of the glucocorticoid receptor prior to activation in the previously elusive step, where it is regulated by the FKBPs’, adds Sarah Engel, key second author of the publication.
These studies, which were funded in part by the LOEWE consortium TRABITA, show how FKBP51 and FKBP52 differentially interact with the glucocorticoid receptor, explain the differentiated pharmacology of FKBP51 ligands, and provide a structural basis for the development of FKBP ligands with higher efficacy. This opens new avenues for the discovery of improved drugs for depression, diabetes-induced obesity, or chronic pain.
