To determine structure parameters – in particular from anisotropic NMR observables – we use so-called polymeric alignment media as an important tool in NMR spectroscopy. The concept relies on the polymers constituting mesogens and forming a lyotropic liquid crystalline phase (LLC) which becomes ordered (aligned) when introduced into a magnetic field. The resulting orientational anisotropy is then transferred to a dissolved compound to be investigated. The nature of these mesogens is responsible for the strength of the anisotropy induced and the structure parameters determined for the investigated compound. We employ lyotropic liquid crystalline systems based on (homo-)polypeptides(homo-)polypeptides and supramolecular polymers of 1,3,5-benzenetricarboxamides (BTAs) for these tasks.
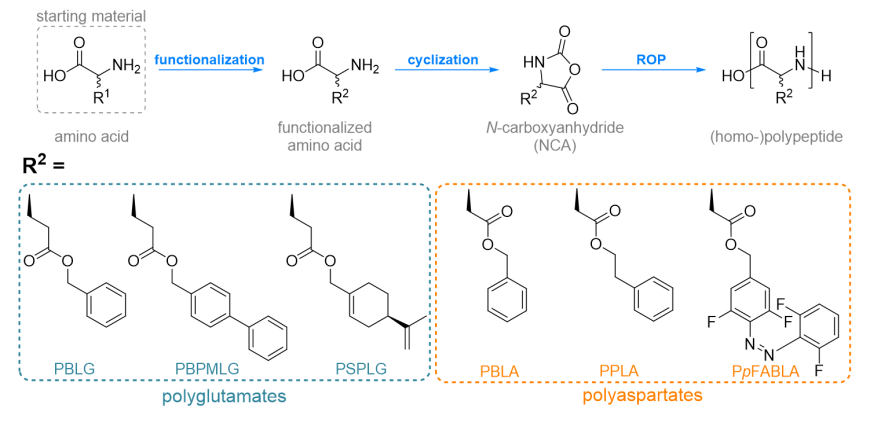
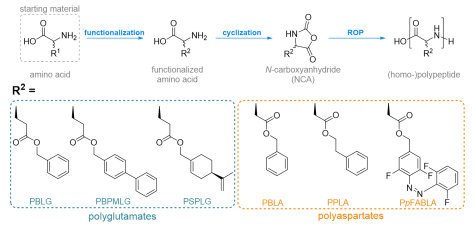
For polymeric alignment media, we synthesize helical, side-chain functionalized polyglutamates (PBLG, PBPMLG , PSPLG) and polyaspartates (PBLA, PPLA, PpFABLA), from the respective starting amino acid. As a first step, the amino acid is substituted with the desired sidechain, followed by the cyclization to the N-carboxyanhydride, which finally is polymerized to the respective polypeptides by ring-opening polymerization (ROP). The impact of the targeted sidechain functionalization with e.g. conformationally flexible or stimuli-responsive moieties on the properties of the synthesized polymers are investigated by a multitude of methods. Isotopic labelling (with 2H) enables analysis of the polymer’s conformation in lyotropic liquid crystalline (LLC) phases and their ordering in the magnetic field via NMR spectroscopy. Complementary information regarding the (helical) chirality are obtained from circular dichroism (CD) spectroscopy, lead to a comprehensive view of the properties of the (stimuli-responsive) polymeric alignment media. This contributes to a more in-depth understanding of the interactions of dissolved compounds with the sidechain substituted polypeptides as alignment media in structure determination projects. In the future, we aim to develop specifically tailored alignment media.
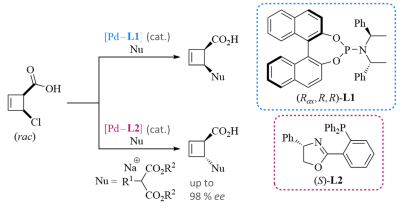
In addition to the synthesis of alignment media, we also investigate (meta-stable) intermediates in thePd-catalyzed allylic alkylation reactions . These should provide insight into the reaction mechanism – particularly into the reasons for the reaction’s stereoselectivity. The intermediates are synthesized as part of the mechanistic investigations, their connectivity and three-dimensional structure is determined by NMR spectroscopy, complemented by DFT calculations.
For the characterization and application of alignment media and to more efficiently study mechanistic problems, we continuously develop new measurement methods and setups. New NMR pulse sequences and analysis methods enable us to extract more information about the investigated systems from the experimental data, while LED-based in situ irradiation methods or NMR imaging are employed in the investigations of stimuli-responsive behavior.